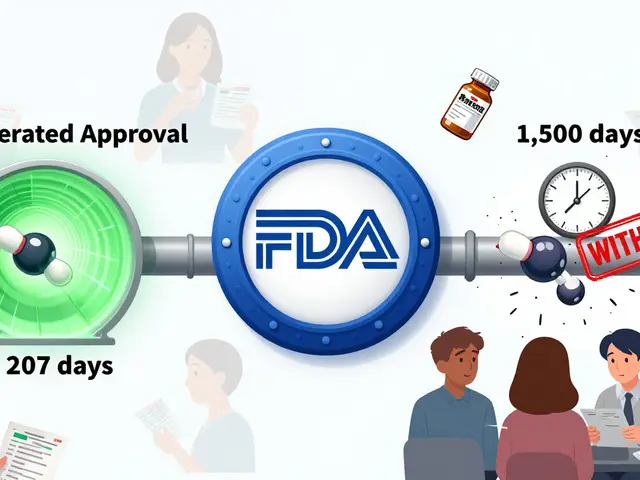
Herbal and Supplement Liver Toxicity: What to Avoid
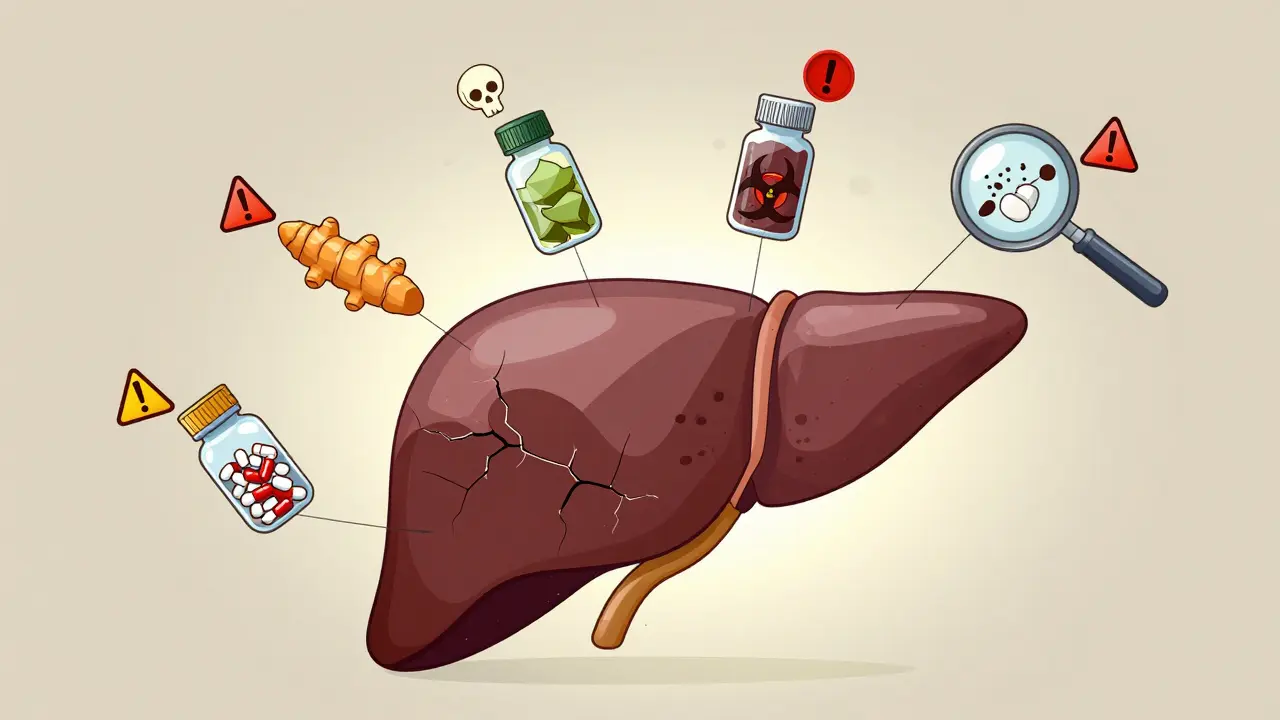
Herbal and supplement liver toxicity is rising fast, with turmeric, green tea extract, and black cohosh among the top culprits. Learn which supplements to avoid and how to protect your liver before it's too late.
CONTINUE