When you hear the word generic, you probably think of cheap pills that work just like the brand-name version. That’s true for most small-molecule drugs. But when it comes to biologics-like drugs for rheumatoid arthritis, cancer, or diabetes-the story changes. Enter biosimilars. They’re not generics. They’re not copies. And confusing them can lead to real misunderstandings about cost, safety, and how your treatment works.
What exactly is a generic drug?
Generic drugs are the simple, straightforward version of brand-name pills. They’re made from single, well-defined chemical compounds. Think ibuprofen, metformin, or aspirin. Once the patent on the original drug expires, any manufacturer can produce the exact same chemical formula. The FDA doesn’t require new clinical trials because the molecule is identical. All they need to prove is that the generic gets into your bloodstream at the same rate and amount as the brand-name version. That’s called bioequivalence.
These drugs are cheap. On average, generics cost 40% to 50% less than their brand-name counterparts. In the U.S., about 90% of all prescriptions filled are generics. They’re in every pharmacy, every insurance formulary, and most doctor’s offices. Pharmacists can swap them in automatically without asking your doctor. That’s because the molecule is identical. No guesswork. No risk of unexpected reactions.
What are biosimilars, really?
Biosimilars are the same kind of cost-saving alternative-but for biologic drugs. These aren’t pills. They’re complex proteins made inside living cells-like yeast, bacteria, or hamster ovary cells. Examples include Humira (adalimumab), Enbrel (etanercept), and Herceptin (trastuzumab). These drugs treat autoimmune diseases, cancer, and chronic conditions. Because they’re made by living systems, no two batches are exactly alike. Even the original manufacturer can’t produce the exact same molecule twice.
A biosimilar isn’t a copy. It’s a highly similar version. The FDA requires manufacturers to prove there are no clinically meaningful differences in safety, purity, or potency compared to the original biologic. That means hundreds of lab tests-analyzing structure, function, stability, and how the body reacts. Animal studies. Sometimes small clinical trials. It’s not just about matching a chemical formula. It’s about matching a biological behavior.
The development cost? Around $100 million to $200 million per biosimilar. Compare that to $2 million to $5 million for a generic. That’s why biosimilars don’t save as much-typically 15% to 33% less than the brand-name drug. Still, that’s billions saved across the healthcare system.
The molecular difference: small molecules vs. big proteins
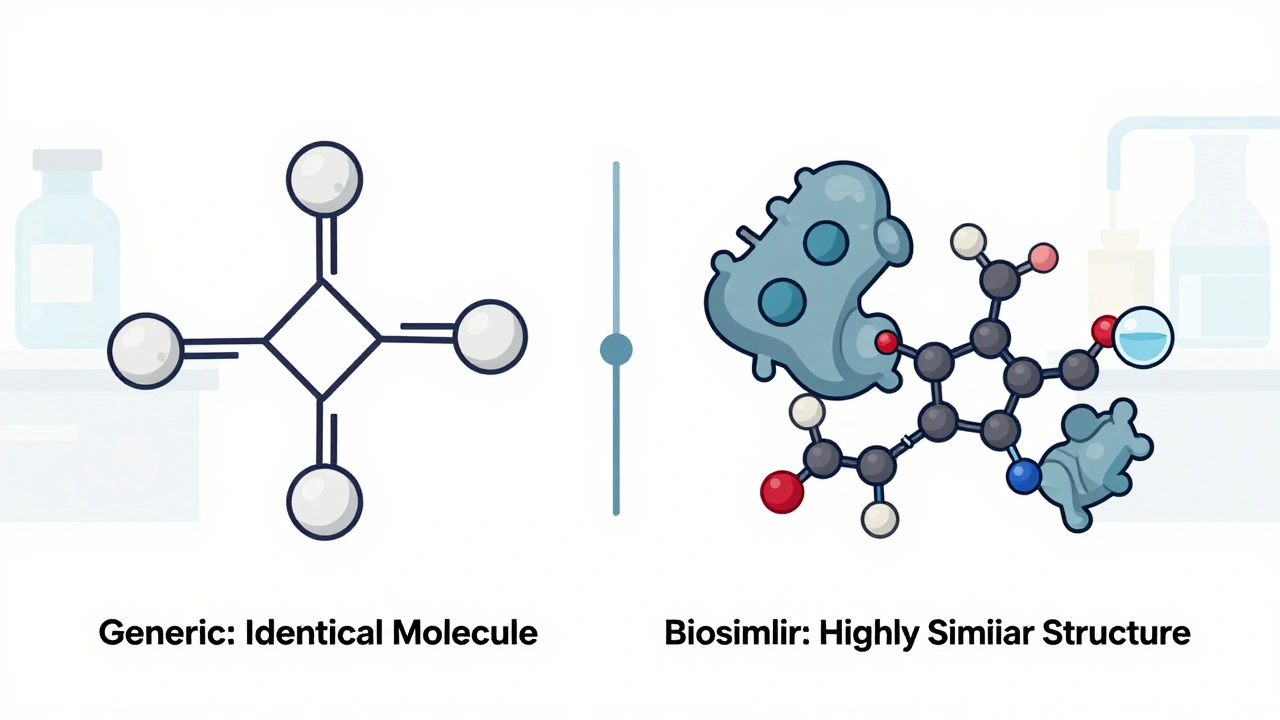
The biggest distinction is size and complexity. A generic drug like aspirin weighs about 180 daltons. A biologic like Humira? About 148,000 daltons. That’s over 800 times heavier. And it’s not just big-it’s intricate. These proteins fold into 3D shapes. They get modified with sugar molecules. They’re sensitive to temperature, pH, and manufacturing conditions. Even tiny changes can affect how the immune system reacts.
Generics are like photocopying a printed page. You get the same text, same font, same ink. Biosimilars are like recreating a hand-painted oil portrait using different brushes, paint, and canvas. The final image looks nearly identical. But if you zoom in under a microscope, you’ll see differences. The key is: those differences don’t change how the painting works.

Regulatory paths: why biosimilars take longer and cost more
Generics follow the Hatch-Waxman Act of 1984. The process is streamlined: prove bioequivalence, done. Biosimilars follow the Biologics Price Competition and Innovation Act (BPCIA) of 2009. That’s a much longer, more complex path. The FDA requires a step-by-step evaluation:
- Structural analysis (what does the molecule look like?)
- Functional testing (how does it bind to targets?)
- Animal studies (is it safe?)
- Pharmacokinetic studies (how does the body absorb and clear it?)
- Immunogenicity assessment (does it trigger unwanted immune responses?)
- Potentially, clinical trials in sensitive patient groups
For generics, you need one or two small studies. For biosimilars, you need dozens. And you can’t just reverse-engineer the original manufacturer’s process. You have to build your own from scratch. That’s why only 42 biosimilars have been approved in the U.S. as of late 2023, compared to over 10,000 generics.
Substitution rules: why your pharmacist can’t swap them automatically
This is where things get tricky. In every U.S. state, pharmacists can substitute a generic drug for a brand-name one without telling your doctor. It’s automatic. That’s not true for biosimilars.
Only biosimilars designated as “interchangeable” can be swapped without a doctor’s approval. And as of 2023, only seven out of 42 approved biosimilars have that status. The rest require a new prescription if your doctor wants to switch you. Why? Because of immunogenicity risk. Even tiny differences in a biologic can cause your immune system to react-leading to reduced effectiveness or allergic responses. The FDA doesn’t assume that all biosimilars are safe to switch to without oversight.
For example, if you’re on Humira and your doctor switches you to a non-interchangeable biosimilar, they need to monitor you closely. If you’re on an interchangeable biosimilar, your pharmacist can swap it without asking. But that’s rare.
Market adoption: why generics dominate and biosimilars are still catching up
Generics make up 90% of prescriptions but only 20% of total drug spending. That’s because they’re so cheap. Biosimilars? They’re less than 3% of the biologics market-even though biologics make up nearly half of all U.S. drug spending. Why the slow uptake?
- Provider hesitation: A 2022 survey found 68% of rheumatologists needed more education before prescribing biosimilars.
- Reimbursement issues: Many hospitals use a “buy-and-bill” model. If they buy a more expensive brand-name drug, they get reimbursed more. Switching to a cheaper biosimilar cuts their profit.
- Patent thickets: Companies like AbbVie filed hundreds of patents on Humira to delay competition. The first biosimilar didn’t hit the market until 2023, even though the patent expired in 2016.
- Less awareness: Patients and providers still confuse biosimilars with generics. Many think they’re the same thing.
But things are changing. The first interchangeable biosimilar for Humira, Amjevita, launched in January 2024 with a 35% discount. The Inflation Reduction Act is also helping by capping insulin costs and closing Medicare Part D coverage gaps. Experts predict biosimilars could capture 25-30% of the U.S. biologics market by 2028.
What this means for patients
If you’re on a generic drug, you’re getting the exact same molecule as the brand. No surprises. No switching risks. If you’re on a biologic, you might eventually be offered a biosimilar. That’s not a downgrade-it’s a cost-saving option backed by rigorous science. But it’s not automatic. You and your doctor need to talk about it.
Here’s what to ask:
- Is my drug a biologic or a small-molecule medication?
- Is there a biosimilar available for it?
- Is the biosimilar designated as interchangeable?
- Will switching affect my treatment plan or insurance coverage?
- Do I need monitoring if we switch?
Don’t assume biosimilars are riskier. They’re not. They’re just different. The FDA requires them to meet the same high safety standards as the original. But they’re not interchangeable with generics. You can’t swap a biosimilar for a generic. They’re made differently, work differently, and are regulated differently.
Bottom line: not the same, but both valuable
Biosimilars and generics both lower drug costs. But they’re not the same. Generics are chemical twins. Biosimilars are biological cousins. One is easy to replicate. The other is incredibly hard to match. One can be swapped at the pharmacy counter. The other needs a doctor’s decision.
Understanding the difference isn’t just academic. It affects your treatment, your out-of-pocket costs, and your long-term health. If you’re on a biologic, ask if a biosimilar could work for you. If you’re on a generic, know that your medication is chemically identical to the brand. And remember: calling a biosimilar a “generic biologic” is wrong-and potentially misleading.
The future of affordable medicine depends on both. But they need to be treated for what they are-not what we wish they were.

15 Comments
Elliot Barrett
This whole post is overkill. I just want my damn insulin to not cost $500. Who cares if it's a biosimilar or generic as long as it works?
Tejas Bubane
Let’s be real - the pharmaceutical industry is running a sophisticated scam. Biosimilars are marketed as ‘innovative cost-savers’ but the truth is they’re engineered to circumvent patent cliffs while maintaining profit margins. The FDA’s ‘no clinically meaningful difference’ standard is a euphemism for ‘close enough for regulatory convenience.’ And don’t get me started on the buy-and-bill model - hospitals are literally incentivized to keep you on the $20,000 drug instead of the $14,000 biosimilar. This isn’t healthcare reform. It’s corporate arithmetic dressed in white coats.
Ajit Kumar Singh
Generics are simple chemistry biosimilars are biology on steroids and honestly people dont get it the molecule is not the point the function is the point and if your immune system reacts differently thats your problem not the drug companies problem also why is everyone so scared of change we used to think vaccines caused autism now we think biosimilars will kill us
Maria Elisha
Ugh I just got switched to a biosimilar last month and my rheumatoid arthritis flared up for two weeks. My doctor said it was ‘just adjustment’ but I’m not buying it. Why risk it when the original works fine? And why can’t my pharmacist just swap it like with my generic metformin? This whole system is a mess.
Angela R. Cartes
Let’s be honest - if you’re not a biochemist or a pharmacist, you shouldn’t be expected to understand the difference between a biosimilar and a generic. The whole system is designed to confuse patients so they don’t question why their insulin still costs $300. The real villain here isn’t the science - it’s the lack of transparency. And yes, I’m using a 💸 emoji because this is literally about money, not medicine.
Andrea Beilstein
What does it mean to be ‘the same’ in a world where biology is inherently variable? A generic aspirin is a static entity - identical in every bottle. But a biologic? It’s a living process. Even the original manufacturer can’t replicate it perfectly. So when we say biosimilars are ‘highly similar,’ are we really saying that similarity is the new truth? Or are we just accepting that in complex systems, perfect fidelity is impossible - and that’s okay? Maybe the real question isn’t whether biosimilars work - but whether we’re ready to accept imperfection in medicine.
Lisa Whitesel
Stop pretending biosimilars are safe. They’re not. The immunogenicity risk is real and doctors are pushing them because they’re pressured by insurers. Patients are being used as guinea pigs. And the FDA? They’re asleep at the wheel. This isn’t innovation - it’s cost-cutting with a fancy label. And if you think it’s okay to swap a biologic without monitoring, you’re either naive or complicit.
Larry Lieberman
So generics = photocopy, biosimilars = hand-painted portrait 🎨 That’s the best analogy I’ve ever heard. Also why is no one talking about how the first interchangeable Humira biosim hit the market with a 35% discount? That’s huge. We’re not just saving money - we’re changing the game. 🚀
Sabrina Thurn
From a clinical pharmacology standpoint, the regulatory pathway for biosimilars is actually more rigorous than for generics - which is why their approval takes longer and costs more. Structural characterization via mass spectrometry, glycosylation profiling, receptor-binding assays, in vitro functional assays, PK/PD modeling, immunogenicity screening - these aren’t just buzzwords. They’re necessary because biologics are inherently more complex. The 15-33% cost reduction isn’t a failure - it’s a reflection of the scientific investment required. And the fact that only 7 are interchangeable? That’s caution, not incompetence. We’re not playing with aspirin here.
Courtney Black
It’s funny how we treat drugs like they’re just chemicals when in reality they’re part of a larger system - of power, profit, fear, and trust. We say ‘biosimilars are safe’ but we don’t say who decided that, or why, or who benefits. We say ‘generics are interchangeable’ but we don’t ask why the same logic doesn’t apply to biologics. We want simplicity in a world that refuses to be simple. Maybe the real issue isn’t the drugs - it’s our need to believe that medicine can be reduced to a formula.
iswarya bala
im so glad biosimilars are coming to india too!! my dad is on humira and we cant afford it anymore. if a cheaper version works its a win. dont overthink it. science is science. 🙏
Simran Chettiar
The distinction between biosimilars and generics is not merely technical, it is epistemological. In the realm of small molecules, identity is ontologically stable - the molecule is the essence. But in biologics, identity is processual - it emerges from the cellular environment, the fermentation conditions, the purification protocols. To equate biosimilars with generics is to impose a mechanistic paradigm upon a phenomenological reality. The regulatory framework must therefore reflect this ontological difference, not merely the economic imperative. The cost differential is not a flaw - it is a necessary consequence of the complexity inherent in biological systems.
Anna Roh
My pharmacist switched me to a biosimilar without telling me. I didn’t even notice until I checked the box. Now I’m paranoid every time I get a refill. Why isn’t there a better system?
Katherine Chan
Big win for patients! Biosimilars are bringing life-changing treatments within reach for so many people who couldn’t afford them before. It’s not perfect, but progress isn’t about perfection - it’s about making things better. Keep pushing for more interchangeable ones and more education. We’ve got this 💪
Olivia Portier
Just had my first biosimilar switch and honestly? No issues. My doc explained everything, we monitored me for 4 weeks, and now I’m saving $1200/month. People freak out over stuff they don’t understand. Talk to your provider. Ask questions. Don’t let fear stop you from saving money and staying healthy 🌱