Let’s cut through the noise: generic drugs aren’t copies in the way you might think. They’re not knockoffs, fakes, or cheap imitations. They’re scientifically proven, FDA-approved versions of brand-name medications that work the exact same way - and save you a fortune.
What Exactly Is a Generic Drug?
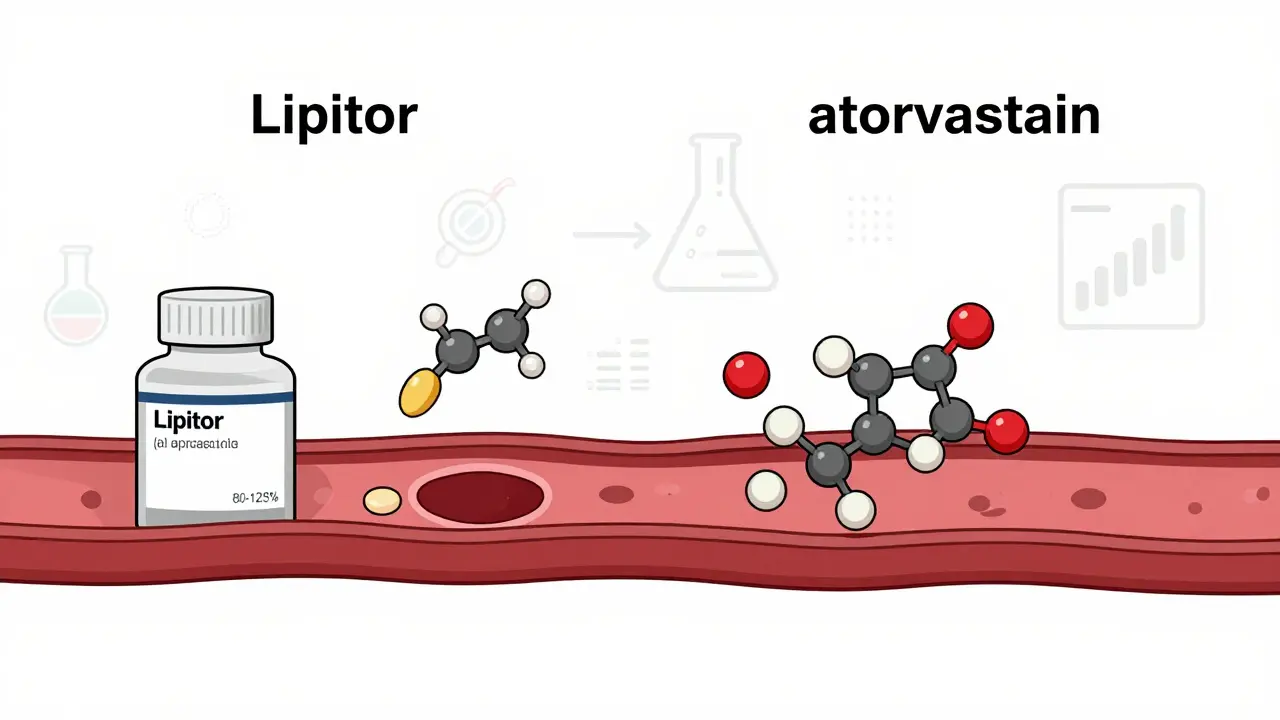
A generic drug contains the same active ingredient, in the same strength, and in the same form as its brand-name counterpart. If you take a brand-name pill called Lipitor for cholesterol, the generic version is atorvastatin. Same molecule. Same effect. Same way it gets into your bloodstream. The U.S. Food and Drug Administration (FDA) doesn’t just approve generics because a company asked nicely. They require proof - hard data - that the generic delivers the same amount of medicine into your body at the same speed as the brand. That’s called bioequivalence. For a generic to be approved, the amount of drug in your blood (measured as AUC and Cmax) must fall within 80-125% of the brand-name drug. That’s not a guess. It’s a lab-tested, math-backed standard. You might notice the pill looks different. Maybe it’s a different color, shape, or has a different logo. That’s because the inactive ingredients - the fillers, dyes, flavors, and binders - can be changed. These don’t affect how the medicine works. But trademark laws require generics to look different from the brand. So yes, your generic pill might be blue instead of purple, but it’s still doing the same job.How Do Generics Get Approved Without Repeating Clinical Trials?
Brand-name drugs go through years of expensive clinical trials to prove they’re safe and effective. That’s why they cost so much. Once the patent expires - usually 20 years after filing - other companies can make the same drug without starting from scratch. The 1984 Hatch-Waxman Act created the Abbreviated New Drug Application (ANDA) process. This lets generic manufacturers prove their version works just like the original by showing bioequivalence, not by running new trials on thousands of patients. The FDA reviews the data, inspects the manufacturing site, and only then approves it. In 2022, the FDA approved over 1,000 new generic drugs. That’s not luck. It’s a system designed to bring down prices without cutting corners on safety.Are Generics Really Just as Effective?
Yes - for the vast majority of drugs. The FDA states clearly: “Generic drugs work the same as brand-name drugs in the same way and provide the same benefit(s).” A 2021 analysis by the Congressional Budget Office found that generic drugs cost, on average, 85% less than brand-name versions. And they work just as well. Think about statins. Over 98% of prescriptions for atorvastatin (generic Lipitor) are filled with the generic. Same for lisinopril (generic Zestril), omeprazole (generic Prilosec), and metformin (generic Glucophage). Millions of people take these every day. Hospitals use them. Medicare uses them. If they didn’t work, we’d know. A 2023 review of 1.2 million patient reviews on Drugs.com showed generics scored 7.2 out of 10 for effectiveness. Brand-name drugs scored 7.5. That’s a tiny difference - statistically meaningless. Most people can’t tell the difference.
When Might Generics Be Different?
There’s one big exception: narrow therapeutic index (NTI) drugs. These are medications where even a tiny change in blood levels can cause serious problems. Too little, and the drug doesn’t work. Too much, and it becomes toxic. Examples include:- Warfarin (blood thinner)
- Levothyroxine (thyroid hormone)
- Phenytoin, carbamazepine (anti-seizure drugs)
Why Do People Think Generics Are Inferior?
Misinformation spreads fast. A Brown University Health survey found that 43% of patients believe generics contain only 20-80% of the active ingredient. That’s completely false. FDA testing shows generics contain 99.2% of the stated active ingredient - right on target. Another myth? That generics are made in shoddy factories. The FDA inspects all manufacturing plants - brand and generic - the same way. In fact, many brand-name companies make their own generics after the patent expires. You’re buying the same product, just without the fancy packaging. Then there’s the placebo effect. If you believe a blue pill is “weaker,” your brain might convince you it is. That’s not the drug’s fault. It’s how we think.How Much Money Do Generics Really Save?
A lot. In 2023, the average generic prescription cost $4.27. The brand-name version? $61.85. That’s over $57 saved per fill. For someone taking three prescriptions a month, that’s $1,700 saved a year. Medicare Part D beneficiaries saved an average of $500+ annually just by using generics. States with laws that allow pharmacists to substitute generics without asking the doctor saw prescription drug costs drop by 12.7%. That’s not a small win. That’s life-changing for people on fixed incomes. And it’s not just about price. A 2022 Harvard study found that 25% of patients skip doses or don’t fill prescriptions because brand-name drugs are too expensive. For generics? That number drops to 8%. Better adherence means better health.
What About Biosimilars? Are They the Same?
Biosimilars are a different category. They’re not “generics” in the traditional sense. Brand-name biologics - like Humira, Enbrel, or Eliquis - are made from living cells. They’re complex, messy molecules. You can’t just copy them like a pill. Biosimilars are highly similar, but not identical. They’re approved through a different process, and they’re still rare - making up less than 5% of the biologic market as of 2023. But they’re growing fast. The FDA is working to speed up approvals, and by 2027, biosimilars could make up 15% of the market. They’re not generics, but they’re the next step in lowering costs for expensive treatments.What Should You Do?
If you’re prescribed a brand-name drug, ask:- Is there a generic available?
- Is it safe for my condition?
- Can I switch without my doctor’s approval?
Final Thoughts
Generic drugs aren’t copies. They’re the same medicine, sold without the marketing budget. They’re tested. They’re approved. They’re used by millions every day. And they’re saving the U.S. healthcare system hundreds of billions of dollars. The myth that generics are inferior isn’t just wrong - it’s expensive. And for people who can’t afford their meds, it’s dangerous. Your health doesn’t care what color the pill is. It only cares if it works. And for 96% of prescriptions, generics do - perfectly.Are generic drugs as safe as brand-name drugs?
Yes. The FDA requires generic drugs to meet the same strict standards for safety, strength, quality, and purity as brand-name drugs. Every generic manufacturer must pass the same inspections and follow the same manufacturing rules. The FDA reviews thousands of generic applications each year and only approves those that prove they’re equivalent.
Why do generic drugs look different from brand-name ones?
U.S. trademark laws require generics to look different from brand-name drugs to avoid confusion. That means different colors, shapes, or markings. But the active ingredient - the part that treats your condition - is identical. The differences are only in inactive ingredients like dyes or fillers, which don’t affect how the drug works.
Can I switch from a brand-name drug to a generic without my doctor’s permission?
In 49 states, pharmacists can substitute a generic for a brand-name drug without asking your doctor - unless your prescription says "dispense as written" or "no substitution." In Mississippi, the doctor must note permission. If you’re on a narrow therapeutic index drug like warfarin or levothyroxine, your pharmacist may check with your doctor first to ensure stability.
Do generic drugs take longer to work?
No. To get FDA approval, a generic must show it delivers the same amount of active ingredient into your bloodstream at the same rate as the brand-name drug. This is proven through bioequivalence studies. If a generic took longer to work, it wouldn’t be approved.
Are all generic drugs made overseas?
Many generic drugs are made overseas, but so are many brand-name drugs. The FDA inspects all manufacturing facilities - whether in the U.S., India, China, or elsewhere - using the same standards. The location doesn’t determine quality. The inspection does. The FDA has inspection sites in over 150 countries.
What should I do if I think my generic drug isn’t working?
Don’t assume it’s the generic. Talk to your pharmacist or doctor first. Sometimes, side effects are caused by inactive ingredients - like a dye or filler - not the active drug. Your pharmacist can check if there’s another generic version available without that ingredient. If you’re on a narrow therapeutic index drug, your doctor may want to check your blood levels. Rarely, a batch issue can occur - but it’s not common.

11 Comments
Tiffany Adjei - Opong
Okay but have you ever tried switching to a generic and felt like your brain was melting? I took the generic omeprazole and suddenly I was having heart palpitations at 3am. Turned out it was the dye. Not the drug. The dye. Who the hell puts red #40 in a stomach pill? 🤡
Wesley Pereira
Let’s be real - the FDA’s 80-125% bioequivalence window is basically ‘close enough for government work.’ If your drug’s Cmax is 124.9%, congrats, you’re ‘equivalent.’ But what if your body metabolizes it at 125.1%? You’re now a liability. And no, I don’t trust the FDA’s inspectors who’ve never seen a real patient. 🧪
Isaac Jules
Stop lying to people. Generics are for peasants. If you can’t afford the brand, maybe you shouldn’t be taking the drug at all. My cousin took a generic antidepressant and ended up in the ER. The FDA doesn’t care. They just want to cut costs. You think your life is worth $4.27? 🤬
Pavan Vora
Dear all, I am from India, where we produce 40% of the world's generics… and yes, some factories are… not perfect. But the Indian regulator, CDSCO, is now following WHO-GMP standards. Also, many US brands outsource to Indian plants - so you're already buying 'generic' quality, just with a fancy label. 🙏
Joann Absi
THEY’RE LYING TO YOU. 🚨 The FDA is owned by Big Pharma. They let generics in so you’ll stop complaining about $60 pills. But here’s the twist: the *same* companies that make Lipitor also make atorvastatin. You’re not saving money - you’re just paying the same people with a different logo. 💸
Ashley S
I tried a generic and it didn't work. So they're fake. End of story. Why would anyone risk their health for a few bucks? I'm not a lab rat.
Gabrielle Panchev
It’s fascinating how the narrative around generics is so deeply rooted in placebo-driven fear-mongering - yet the data, the bioequivalence metrics, the FDA’s own longitudinal studies, and even the meta-analyses from the Cochrane Collaboration consistently show no clinically significant difference in outcomes - unless you’re switching manufacturers every two weeks, which, frankly, is a terrible idea for any drug, generic or not, because your body needs consistency, not marketing slogans - and yes, I’ve read the 2023 Drugs.com review of 1.2 million reviews and the 0.3-point difference is statistically irrelevant, but people still believe what they feel, not what the data says, which is why we have a public health crisis of misinformation masquerading as personal experience - and I’m not even going to get into the fact that the FDA’s inspection backlog has doubled since 2018, which means some plants are flying under the radar, but that’s a separate issue -
Katelyn Slack
My pharmacist switched me to generic metformin and I got a rash. I went back and asked for the original. She said it's the same. But my skin didn't think so. I'm sticking with the purple pill now. 🤷♀️
Melanie Clark
They say generics are safe but have you ever heard of the 2018 valsartan recall? Contaminated with NDMA carcinogen. And guess what? It was a generic. Made in China. The FDA knew. They didn’t tell anyone for months. Now tell me again how safe this system is? 🕵️♀️
Venkataramanan Viswanathan
In India, we call generics 'affordable medicines.' We don't call them 'cheap.' There is dignity in access. If a diabetic in rural Bihar takes insulin because it costs $2 instead of $200, that is not a compromise - that is justice. The West forgets that medicine is a right, not a luxury.
Vinayak Naik
Bro, I work in a pharmacy in Kerala and we ship generics to 12 countries. The stuff we make? Pure as mountain spring. Some US brands? They outsource their generics to us and slap their logo on it. So you’re paying $60 for something we sell for $0.80. The real scam? The brand-name marketing machine. Not the pill. 🤝