Generic Drug Approval: What It Means and How It Keeps Medicines Safe and Affordable
When you see generic drug approval, the process by which regulatory agencies like the FDA confirm that a generic version of a brand-name drug is chemically identical and works the same way in the body. Also known as bioequivalence approval, it’s the gatekeeper that lets you buy the same medicine for a fraction of the price without risking your health. This isn’t just about saving money—it’s about making sure millions can get the treatment they need. Without this system, drugs like metformin, lisinopril, or even common blood thinners would stay out of reach for most people.
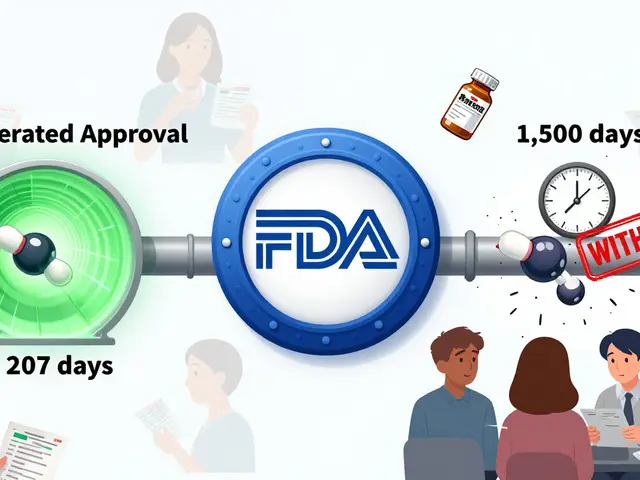
Behind every approved generic is a strict set of tests. The manufacturer must prove the active ingredient matches the brand-name drug in strength, purity, and how fast it dissolves in your body. It doesn’t need to look the same—color, shape, or flavor don’t matter. But the way it works inside you? That has to be identical. The FDA doesn’t repeat full clinical trials for generics. Instead, they rely on bioequivalence studies—comparing blood levels of the drug in healthy volunteers. If the generic delivers the same amount of medicine at the same speed, it gets the green light. And yes, this applies to complex drugs too, like those used for epilepsy, heart failure, or autoimmune conditions. You’ll find real-world examples in posts about generic Lamictal, a generic version of the anticonvulsant lamotrigine used for seizures and bipolar disorder, or generic Zyrtec, the over-the-counter antihistamine cetirizine sold under many names.
But not all cheap drugs are created equal. Some sellers skip approval entirely, selling fake or poorly made versions online. That’s why knowing the difference between a biosimilar, a highly similar but not identical copy of a biologic drug, often used for cancer or autoimmune diseases and a true generic matters. Biosimilars need more testing because they’re made from living cells, not chemicals. Generics are simpler—they’re exact chemical copies. The posts here cover both, from how drug safety, the ongoing monitoring of side effects and effectiveness after approval works, to how to spot a trustworthy pharmacy. You’ll also see how approval affects real decisions, like choosing between generic drugs, affordable versions of brand-name medications that undergo the same strict approval process and insurance-covered options, or why some people pay more at the pharmacy even when a generic exists.
What you’ll find below isn’t just theory. It’s people navigating real choices: saving hundreds on antihistamines, understanding why a generic blood thinner might be safer than stopping your medication, or learning how to tell if that cheap online pill is legit. Every post ties back to one thing: knowing how drugs get approved helps you use them better, safer, and smarter.