When your doctor talks about switching from a brand-name drug to a cheaper version, you might hear the words biosimilar or generic. At first glance, they sound like the same thing: a lower-cost alternative. But they’re not. Confusing them could mean missing out on savings-or worse, making a choice that doesn’t fit your treatment needs.
Let’s cut through the noise. Generics and biosimilars both save money. But how they’re made, how they work in your body, and how they’re regulated are worlds apart. If you’re taking a drug for rheumatoid arthritis, cancer, diabetes, or another chronic condition, knowing the difference isn’t just helpful-it’s critical.
What’s a Generic Drug?
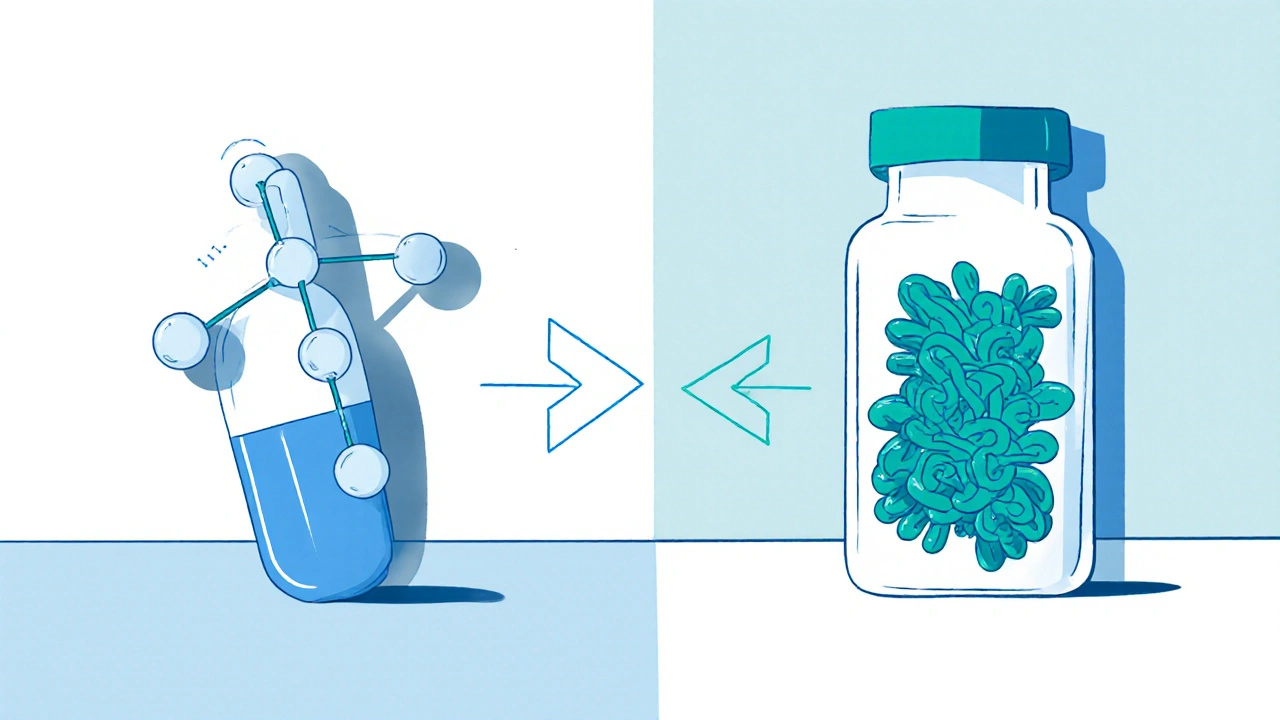
Generics are copies of small-molecule drugs. Think of them like a photocopy of a simple sketch. The original drug, say, atorvastatin (Lipitor), has a chemical structure that’s easy to replicate exactly. Generics contain the same active ingredient, in the same dose, taken the same way. They’re made through chemical synthesis in a lab, not from living cells.
The FDA requires generics to prove they’re bioequivalent to the brand-name version. That means your body absorbs them at the same rate and to the same extent. In 97% of cases, the results are identical. A 2019 JAMA study looked at 47 trials involving heart medications and found no difference in outcomes between brand-name drugs and their generics.
Because they’re simpler to make, generics cost 80-85% less than the original. A month’s supply of generic levothyroxine might cost $10 instead of $70. That’s why over 90% of prescriptions in the U.S. are filled with generics. In most states, your pharmacist can swap a brand-name drug for a generic without asking you-unless your doctor specifically says not to.
What’s a Biosimilar?
Biosimilars are different. They’re copies of biologic drugs-medicines made from living cells, like proteins, antibodies, or enzymes. These aren’t simple molecules. They’re complex, three-dimensional structures, like a snowflake made of protein. Even tiny changes in the manufacturing process-temperature, cell line, purification method-can alter the final product.
That’s why biosimilars aren’t exact copies. They’re highly similar. The FDA requires extensive testing: thousands of lab analyses, animal studies, and clinical trials to prove there’s no meaningful difference in safety or effectiveness. But because of the complexity, they can’t be 100% identical.
Examples include biosimilars for adalimumab (Humira), infliximab (Remicade), and trastuzumab (Herceptin). These treat autoimmune diseases and cancer-conditions where no small-molecule generic exists. Biosimilars typically save 15-20% compared to the original biologic. That’s less than generics, but still massive when the original drug costs $20,000 a year.
Here’s the catch: not all biosimilars are interchangeable. Only those that have passed extra testing to prove you can switch back and forth between the original and the biosimilar safely can be swapped at the pharmacy. Even then, 28 states require your pharmacist to notify your doctor within 72 hours of the switch.
Why the Difference Matters for Your Treatment
Let’s say you’re on insulin for type 1 diabetes. Your doctor prescribes Lantus. You’re offered a biosimilar-Semglee. It’s approved as interchangeable, meaning your pharmacist can switch you without asking. The active ingredient is nearly identical. Your blood sugar stays stable. You save $500 a month.
Now imagine you’re on Humira for psoriatic arthritis. Your doctor switches you to an adalimumab biosimilar. You feel fine. But a few months later, you start having flare-ups. Is it the biosimilar? Or stress? Or something else?
Here’s the reality: for most people, biosimilars work just as well. A 2022 review of 128 studies involving over 38,000 patients found no difference in effectiveness or side effects between Humira and its biosimilars. But some patients-especially those with inflammatory bowel disease-report anxiety about switching. That’s not irrational. It’s human.
The key isn’t fear. It’s understanding. If your treatment is stable and you’re doing well, switching to a biosimilar can be safe and smart. But if you’ve had bad reactions in the past, or your condition is unstable, talk to your doctor before making any changes.
Cost, Access, and Real-World Challenges
Generics are everywhere. They’re stocked in every pharmacy. Insurance plans push them hard. You pay a $10 copay. Done.
Biosimilars? It’s messier. Even though they’re approved and cheaper, many doctors don’t prescribe them. A 2023 AMA survey found only 58% of non-specialists felt confident prescribing biosimilars. Why? Lack of training. Confusion over state laws. Fear of patient backlash.
Insurance formularies also play a role. Some plans still list the brand-name biologic as preferred, even if a biosimilar is cheaper. Others require prior authorization-more paperwork, more delays. In oncology, where biosimilars have been adopted fastest, 83% of filgrastim prescriptions are now biosimilars. In rheumatology? Only 42% for Humira biosimilars.
And then there’s the device issue. A biosimilar insulin might come in a different pen than the original. For elderly patients or those with limited dexterity, that can lead to dosing errors. One Reddit user shared: “My mom switched to Basaglar and kept pressing the wrong button. She ended up underdosing for weeks.”
Manufacturers are starting to help. Amgen, Pfizer, and others offer support programs with nurse hotlines, financial aid, and training on how to use the device. But you have to ask for it.
What You Can Do Right Now
Here’s a simple checklist to help you make the right call:
- Know your drug. Is it a small molecule (pill, tablet) or a biologic (injection, infusion)? If it’s a pill, it’s likely a generic candidate. If it’s an injection, it’s probably a biologic-and a biosimilar may be available.
- Ask your pharmacist. When you get your prescription, ask: “Is there a generic or biosimilar version?” They can tell you what’s covered and what’s interchangeable.
- Check your insurance. Log into your plan’s website. Search your drug name. See what’s on the formulary. Look for “preferred” or “step therapy” notes.
- Talk to your doctor. Don’t assume they know all the options. Say: “I’m interested in saving money. Is there a biosimilar or generic that works for my condition?”
- Monitor your response. If you switch, keep track of symptoms, side effects, and how you feel. Report changes to your doctor-even if they seem minor.

What’s Changing in 2025
The landscape is shifting fast. In July 2024, the FDA approved the first biosimilar for ustekinumab (Stelara), a major drug for psoriasis and Crohn’s disease. That could save patients over $5 billion a year in the U.S. alone.
The Inflation Reduction Act of 2022 removed financial penalties for doctors who prescribe biosimilars in Medicare Part B. That’s pushed hospitals to adopt them faster. Now, 89% of U.S. hospitals use biosimilar infliximab.
Interchangeable biosimilars are growing too. Cyltezo, the first interchangeable biosimilar for Humira, hit the market in August 2023. More are coming. By 2027, experts predict biosimilars will make up 45% of all biologic prescriptions-up from 22% today.
But patent battles still delay entry. A single biologic drug can face over 100 patent claims, pushing biosimilars out by years. The FTC is cracking down, but progress is slow.
Bottom Line: Choose Based on Science, Not Assumptions
Generics are the workhorse of affordable medicine. They’re safe, effective, and widely available. If your drug is a generic, take it. No hesitation.
Biosimilars aren’t the same. They’re not cheaper across the board. They’re not always interchangeable. But for biologics-where there’s no generic alternative-they’re the best option we have. They’ve been studied in tens of thousands of patients. The data says they work.
Don’t let fear or confusion stop you from saving money. But don’t switch blindly either. Ask questions. Get the facts. Work with your care team. Whether it’s a pill or a shot, the goal is the same: effective treatment at a price you can live with.



11 Comments
Kihya Beitz
So let me get this straight - we’re supposed to trust a ‘highly similar’ protein snowflake that costs 15% less and might come in a pen that doesn’t click right? 🤡 My grandma switched to a biosimilar insulin pen and ended up giving herself half a dose for three weeks. She thought the button was for ‘pause.’ Not a joke. Now she’s on a feeding tube. Thanks, capitalism.
Jennifer Walton
Identity in medicine is a construct. The molecule is not the self. The body does not know brand names. Only the fear of the unknown creates the illusion of difference.
BABA SABKA
Bro, you're talking about pharmacokinetics like it's a religious text. Biosimilars aren't 'copies' - they're engineered alternatives with the same functional epitopes. The FDA doesn't approve snake oil. If you're still scared of biosimilars, you're either a pharma shill or you've never read a single clinical trial. The data is there. Stop being a Luddite.
Chris Bryan
They’re pushing these ‘biosimilars’ because Big Pharma and the CDC are in bed with the WHO. You think they want you to get better? Nah. They want you dependent on cheap meds so they can track your biometrics through your insulin pen. That’s why the new pens have ‘smart’ chips. They’re not for dosing - they’re for surveillance. Read the fine print. The FDA didn’t test for that.
Jonathan Dobey
Oh, so now we’re commodifying life itself? A biosimilar isn’t just a cheaper version - it’s a metaphysical compromise. The biologic is a symphony composed by nature; the biosimilar is a MIDI file played on a Casio. Sure, the notes are similar - but the soul? The soul is lost in the purification process. We’re not just saving money. We’re sacrificing the sacredness of biological complexity for a 15% discount. And you call that progress? 🤔
ASHISH TURAN
As someone from India where generics have been the backbone of healthcare for decades, I can say this: the fear around biosimilars is mostly cultural. We’ve been using generics since the 80s. No mass casualties. No secret side effects. Just people living longer, cheaper. The real issue isn’t science - it’s marketing. Big pharma sold us the myth that ‘brand = better.’ It’s time to unlearn that.
Ryan Airey
Everyone’s acting like biosimilars are some dangerous experiment. Newsflash: they’ve been used in Europe since 2006. Over 2 million patients. Zero clinically meaningful differences. The only people panicking are the ones getting paid to scare you. Your doctor’s hesitation? That’s not concern - that’s ignorance. Demand the biosimilar. If they resist, get a new doctor. This isn’t rocket science.
Hollis Hollywood
I just want to say - I switched to a Humira biosimilar last year after reading all the studies. I was terrified. I kept a journal. I tracked my joint pain, my sleep, even my mood. For the first month, I felt weird - but not because of the drug. Because I was scared. And then, slowly, I realized: I felt the same. Same energy. Same flare-ups (or lack thereof). I didn’t need to be afraid. I just needed to be informed. And honestly? I saved $400 a month. That’s my kid’s piano lessons. That’s peace of mind. I’m not a hero. I just listened. And I’m so glad I did.
Aidan McCord-Amasis
Generics = good. Biosimilars = also good. Stop overthinking it. 🤷♂️💸
Adam Dille
My cousin’s on a biosimilar for RA and she’s doing amazing. The pen was weird at first - but Amgen sent her a video tutorial and a free nurse call. No big deal. Honestly? The system’s broken, but the meds? They work. We just need to stop treating patients like lab rats and start treating them like people who just want to feel better without going broke. 🙏
Katie Baker
Thank you for writing this. I’ve been scared to ask my doctor about switching because I didn’t want to seem cheap. But you’re right - it’s not about being cheap. It’s about being smart. I’m going to print this out and take it to my next appointment. You’ve given me the courage to ask. 💪