ADR Reporting: What It Is and Why It Saves Lives
When a drug causes harm instead of healing, that’s an adverse drug reaction, an unintended and harmful response to a medicine at normal doses. Also known as ADR, these reactions range from mild rashes to life-threatening organ failure — and they’re often missed until it’s too late. That’s where ADR reporting, the system doctors and patients use to flag unexpected drug side effects comes in. It’s not just paperwork. It’s the early warning system that keeps millions safe.
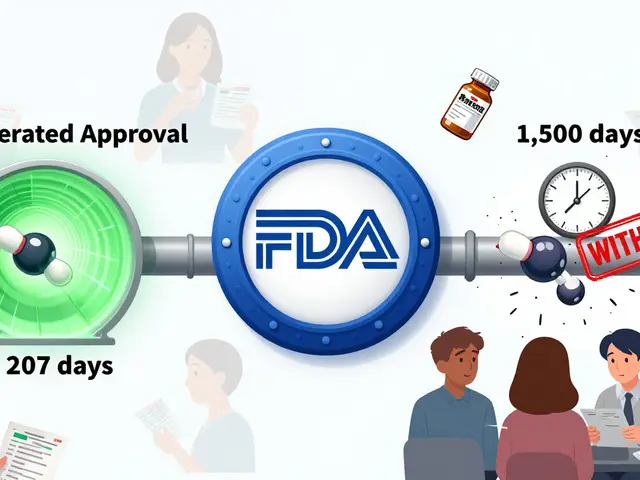
Every time someone reports an ADR — whether it’s a patient noticing strange fatigue after starting a new pill, or a nurse spotting liver damage in a hospital ward — that data feeds into a global network called pharmacovigilance, the science of detecting, assessing, and preventing drug-related harm. Agencies like the FDA and EMA use these reports to spot patterns. One report might mean nothing. But 50 reports of the same rare reaction? That’s a red flag. That’s how drugs get new warnings, dosing limits, or even pulled from the market. Think of it like a crowd-sourced safety net for medicine.
ADR reporting doesn’t just protect patients. It helps doctors choose better treatments. If a common blood pressure drug keeps showing up in reports as causing severe dizziness in older adults, prescribers can switch to something safer. It also reveals hidden risks — like how a popular antibiotic might trigger heart rhythm problems in people with kidney disease, something not caught in clinical trials because those patients were excluded. These aren’t hypotheticals. Real people, real data, real outcomes.
You don’t need to be a doctor to report an ADR. If you or someone you know had an unexpected reaction to a medication — even if you’re not sure it was the drug — it’s worth reporting. Hospitals, pharmacies, and online portals make it simple. The more reports, the clearer the picture. And that picture? It’s what keeps the next person from ending up in the ER because a side effect was ignored.
Below, you’ll find real-world examples of how ADR reporting has changed drug safety, what to look for when something feels off after starting a new medicine, and how systems like the FDA’s SrLC database track these alerts over time. These aren’t theory pieces. They’re lessons learned from actual cases — the kind that shaped how we use meds today.