Adverse Event Reporting: What It Is and Why It Saves Lives
When a medication causes an unexpected or harmful reaction, that’s called an adverse event reporting, the system used to collect and analyze harmful reactions to medicines after they’re on the market. It’s not just paperwork—it’s the last line of defense between a drug and public harm. Every time someone reports a bad reaction—whether it’s a patient, doctor, or pharmacist—they’re helping regulators spot patterns no clinical trial could catch.
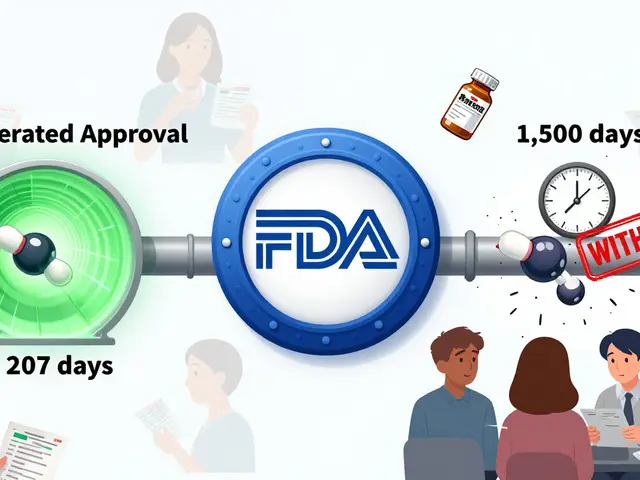
Pharmacovigilance, the science of monitoring drug safety after approval is built on this. It’s how we learned that certain blood thinners increase bleeding risk in older adults, or why some antibiotics can cause dangerous heart rhythms. The FDA reporting, the U.S. system where healthcare providers and patients submit safety data isn’t perfect, but it’s the most powerful tool we have to catch problems early. Without it, drugs like Vioxx or fen-phen might still be on shelves.
These reports don’t just come from hospitals. A patient who gets a rash after starting a new pill, a nurse who notices unusual dizziness in a senior on multiple meds, or a pharmacist who sees the same side effect pop up three times this week—all of these count. And they’re all part of a bigger picture. The more people report, the faster problems get found. That’s why adverse event reporting isn’t just for experts. It’s for anyone who takes medicine.
What you’ll find in the posts below are real examples of how safety systems work in practice. From how boxed warnings get updated to how bioequivalence studies help prevent dangerous generic drug variations, each article shows how safety isn’t just a policy—it’s a process built on real data from real people. You’ll see how drug labels change, how side effects are tracked over time, and why some medications need extra monitoring. This isn’t theory. It’s what keeps you safe every time you fill a prescription.