When you pick up a generic pill at the pharmacy, you expect it to work just like the brand-name version. But how do regulators know it’s truly the same? The answer lies in two numbers: Cmax and AUC. These aren’t just lab jargon-they’re the backbone of bioequivalence, the science that ensures generics are safe and effective replacements.
What Cmax and AUC Actually Measure
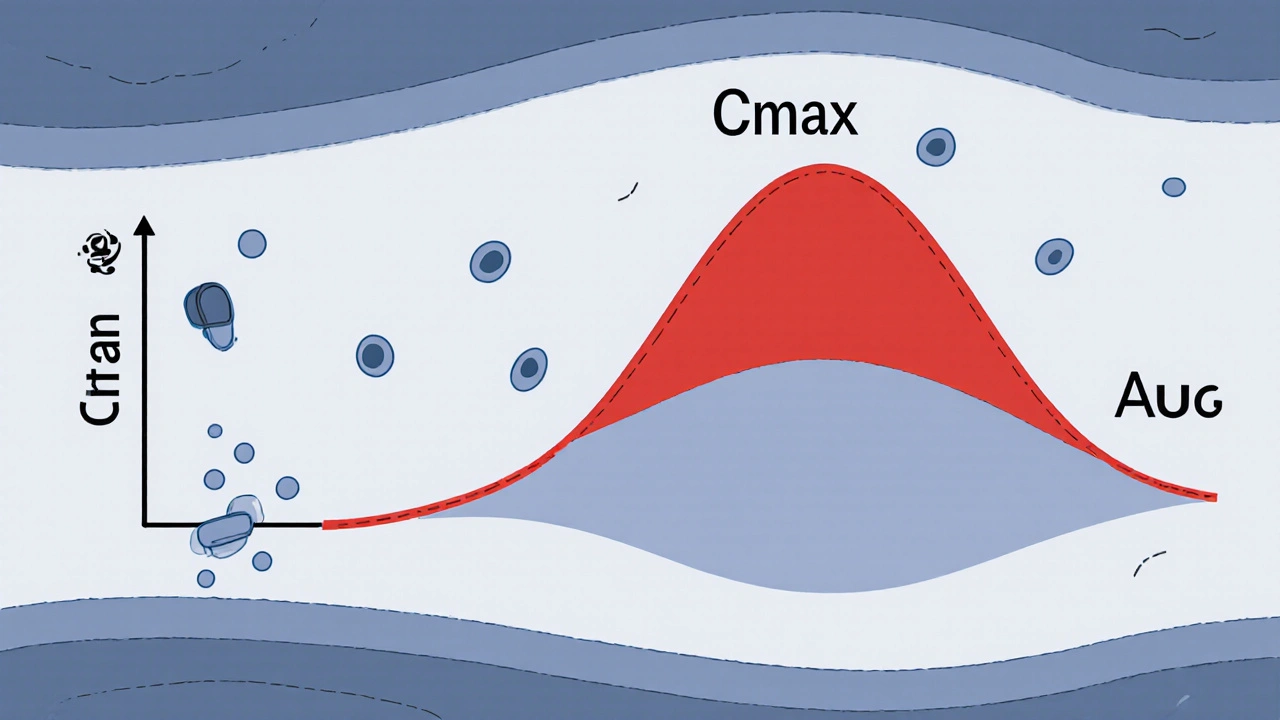
Cmax stands for maximum concentration. It’s the highest level of drug found in your bloodstream after you take a dose. Think of it like the peak of a wave-how high the drug rises in your blood. AUC, or area under the curve, measures the total amount of drug your body is exposed to over time. It’s the entire shape of the wave, from when it starts rising until it fades away.
For example, if you take a painkiller, Cmax tells you how fast and how high the drug hits your system-critical if the drug works best when it peaks quickly. AUC tells you how much total drug your body absorbs over hours or days. If you’re on a blood thinner like warfarin, even a small drop in AUC could mean your blood doesn’t thin enough. A spike in Cmax could cause dangerous bleeding.
These numbers are measured in real studies. Healthy volunteers take the drug, and blood samples are drawn every 15 to 60 minutes for up to 48 hours. Labs use ultra-sensitive machines-LC-MS/MS-that can detect drug levels as low as 0.1 nanograms per milliliter. That’s like finding a single drop of ink in an Olympic-sized swimming pool.
Why Both Numbers Matter-Not Just One
Some people think if AUC matches, Cmax doesn’t matter. That’s wrong. Both are required by law.
Take a fast-acting insulin or a seizure medication. If the generic version reaches peak concentration too slowly, it won’t control blood sugar or prevent seizures in time. Even if the total exposure (AUC) is identical, the delay in peak could mean a seizure happens during the lag. That’s why regulators look at Cmax.
On the flip side, a drug like digoxin, used for heart rhythm, has a very narrow safety window. Too much in your blood (high Cmax) can cause poisoning-even if the total exposure (AUC) is fine. That’s why you can’t ignore Cmax.
The U.S. FDA and the European Medicines Agency both say: both Cmax and AUC must pass. One failing means the whole study fails. No exceptions. This isn’t just a formality-it’s based on decades of clinical data showing that if either number is off, patients can have worse outcomes.
The 80%-125% Rule: How Much Difference Is Allowed?
Here’s the golden rule: the ratio of the generic’s Cmax and AUC to the brand-name drug must fall between 80% and 125%. That means if the brand’s AUC is 100, the generic’s must be between 80 and 125. Same for Cmax.
This range isn’t random. It comes from statistics. Pharmacokinetic data (like drug levels in blood) don’t follow a normal bell curve-they follow a log-normal curve. So scientists transformed the numbers using logarithms. The 80-125% range became a symmetrical ±0.2231 on the log scale. That’s the math behind the rule.
Why not 90-110%? Because research shows that a 20% difference in exposure rarely affects how a drug works for most people. A 2019 analysis of 42 studies in JAMA Internal Medicine found no real difference in safety or effectiveness between generics and brand-name drugs that met this 80-125% standard.
But there are exceptions. For drugs like warfarin, levothyroxine, or cyclosporine-where even a 5% change can cause harm-regulators now use tighter limits: 90-111%. These are called narrow therapeutic index drugs. For them, the stakes are higher, so the rules are stricter.

How Bioequivalence Studies Are Done
A typical bioequivalence study involves 24 to 36 healthy adults. They’re split into two groups. One group takes the brand-name drug first, then the generic after a washout period. The other group does the reverse. This is called a crossover design-it removes individual differences in metabolism.
Blood is drawn 12 to 18 times over 24 to 72 hours, depending on the drug’s half-life. Missing even one key sample during the first two hours can ruin the Cmax estimate. In fact, poor sampling in the absorption phase is the #1 reason studies fail.
Once the data is collected, it’s analyzed using specialized software like Phoenix WinNonlin. The numbers are log-transformed, ratios are calculated, and 90% confidence intervals are built. Only if both AUC and Cmax fall within 80-125% is the product approved.
In 2022, over 1,200 generic drugs were approved in the U.S. alone-every single one required this exact process. The global market for these studies is worth over $2 billion, and it’s growing fast.
What Happens When the Numbers Don’t Match?
Not every generic passes. If a study fails, the manufacturer must go back to the drawing board. They might change the tablet’s coating, the particle size, or the manufacturing process. Sometimes, the generic isn’t even submitted for approval.
There’s also the case of highly variable drugs-those where the same person’s drug levels jump around a lot from day to day. For these, the standard 80-125% rule can be too strict. A generic might be perfectly safe and effective, but just happen to show lower Cmax on one test day. To fix this, the EMA allows something called scaled average bioequivalence. It adjusts the acceptance range based on how variable the drug is. The FDA allows it too, but only for certain drugs and with extra proof.
Still, most drugs don’t need this. For the vast majority, the classic Cmax and AUC method works perfectly.
Why This Matters to You
You don’t need to understand the math. But you should know this: every generic you take has been tested against the brand using these two numbers. That’s why you can trust it.
There’s no evidence that generics meeting these standards are less effective or more dangerous. In fact, studies show they perform the same in real-world use. The reason generics cost less isn’t because they’re inferior-it’s because they don’t need to repeat the billion-dollar clinical trials the original drug went through.
And if your doctor switches you to a generic? You’re not getting a second-rate drug. You’re getting one that’s been proven, down to the last nanogram, to behave the same in your body.
What’s Next for Bioequivalence?
Scientists are exploring new ways to measure drug exposure, especially for slow-release or complex formulations. Some are looking at partial AUC-focusing only on the first few hours for drugs that act quickly. Others are using computer models to predict how a drug will behave without running full human studies.
But for now, and for the foreseeable future, Cmax and AUC remain the gold standard. They’ve been used for over 40 years. They’ve been validated in millions of patients. They’ve saved billions in healthcare costs. And they’ve kept patients safe.
So next time you see a generic on your prescription, remember: behind that label is a science built on two simple numbers-and a whole lot of careful measurement.
What does Cmax mean in bioequivalence?
Cmax stands for maximum plasma concentration-the highest level a drug reaches in your bloodstream after you take it. It tells you how fast the drug is absorbed. For drugs that need to act quickly, like painkillers or seizure meds, Cmax is critical. If the generic’s Cmax is too low or too high compared to the brand, it could mean the drug doesn’t work right or causes side effects.
What does AUC mean in bioequivalence?
AUC, or area under the curve, measures total drug exposure over time. It’s calculated by plotting drug concentration in your blood against time and measuring the area under that curve. AUC tells you how much of the drug your body absorbs overall. For drugs that work over hours, like antibiotics or antidepressants, AUC is the best predictor of effectiveness.
Why do regulators require both Cmax and AUC?
Because they measure different things. Cmax tells you about the rate of absorption-how fast the drug gets into your blood. AUC tells you about the extent of absorption-how much total drug your body gets. A drug could have the same total exposure (AUC) but reach peak levels too slowly or too quickly (Cmax), which could affect how well it works or if it causes side effects. Both must pass to ensure the generic is truly equivalent.
What is the 80%-125% rule in bioequivalence?
The 80%-125% rule is the legal acceptance range for the ratio of generic to brand-name drug levels. For a generic to be approved, the 90% confidence interval of its geometric mean Cmax and AUC must fall between 80% and 125% of the brand-name drug. This means the generic can’t be more than 25% higher or 20% lower in exposure. This range was chosen because studies show a difference within this range rarely affects safety or effectiveness for most drugs.
Are there drugs that need stricter bioequivalence limits?
Yes. Drugs with a narrow therapeutic index-like warfarin, levothyroxine, cyclosporine, and digoxin-require tighter limits: 90%-111%. Even small changes in exposure can lead to serious side effects or treatment failure. For these drugs, regulators apply stricter standards because the margin for error is tiny.
Do bioequivalence studies always use healthy volunteers?
Most do-because they need to isolate how the drug behaves without interference from disease. But for drugs meant for specific populations (like children or elderly patients), studies may be done in those groups. However, for standard generics, healthy volunteers are the norm. They’re easier to monitor, and their metabolism is more predictable, making the results more reliable.
Can a generic fail bioequivalence testing?
Yes. If the 90% confidence interval for either Cmax or AUC falls outside 80%-125%, the study fails. Common reasons include poor sampling timing, incorrect formulation, or manufacturing inconsistencies. When this happens, the company must reformulate the drug and run a new study. About 10-15% of initial studies fail, mostly due to inadequate blood sampling during the absorption phase.


15 Comments
Jacob Keil
Cmax and AUC? Sounds like some fancy lab crap to make us feel better about paying less for pills. I don't care if the numbers match-I just want my headache to go away. And if it doesn't? Blame the generic. Not the system.
Andrea Jones
Okay but can we talk about how wild it is that we trust a pill to work based on a curve and a peak? Like… we’re literally betting our health on statistical magic. And yet, it works. 🤯 Thanks, science. Even if I don’t understand half of it.
Justina Maynard
Let’s be real-this whole system exists because Big Pharma doesn’t want generics to undercut them, so they invented a 40-year-old math trick to pretend they’re equal. The 80-125% rule? That’s not science. That’s a compromise wrapped in a lab coat. And don’t get me started on how they pick the ‘healthy volunteers’-usually college kids on meal plans.
Evelyn Salazar Garcia
Why do we even need this? America makes the best drugs. Everything else is just a copycat.
Phil Thornton
Genius. Just… genius. The fact that we can measure a drop of ink in an Olympic pool? That’s the kind of precision that keeps people alive. Respect.
Sean Slevin
Wait-so… you’re telling me… that… the entire… regulatory framework… for generics… is based on… logarithmic transformations… of… pharmacokinetic data… that… follows… a… log-normal… distribution… and… not… a… normal… one…?!
Oh my god. I think I just had a spiritual awakening. I’ve been taking generics for 12 years… and I never knew… the math… was… this… beautiful…
Someone… get this man… a Nobel…
DENIS GOLD
Europe lets them use ‘scaled average bioequivalence’? That’s just giving up. We don’t bend the rules in America. We crush the competition. That’s why our drugs are better.
gina rodriguez
This was so clearly written. I’m not a scientist, but I finally get why my doctor says generics are safe. Thank you for explaining it without making me feel dumb.
Sue Barnes
You people act like this is some sacred science. It’s not. It’s a loophole. The FDA approves generics because they’re cheaper-not because they’re better. And don’t even get me started on the fact that most studies are done in healthy young men. What about women? Elderly? People with real diseases?
jobin joshua
Bro… this is next level 🤯 I didn’t know pills had to pass math tests 😂
Also… can someone explain why my Indian generic works better than my US one? 🤔
Sachin Agnihotri
Man… I just took a generic for my blood pressure… and now I’m reading this… and I’m like… wow… I didn’t realize… this much science… went into… my little white pill…
Thanks for making me feel… less dumb… today…
Diana Askew
Of course they say it's safe. They're lying. The FDA is owned by Big Pharma. They let generics in so they can charge more for the brand. The real drugs are kept in vaults. That’s why your insurance won't cover the brand-it's a scam.
And those 'healthy volunteers'? They're all paid actors. I know a guy who works at a clinical trial site.
Pawittar Singh
Bro this is dope! 🙌 Seriously-this is the kind of stuff that should be taught in high school. We need more people who understand how medicine actually works-not just the ads. Keep sharing this. We’re lucky to have science like this.
Josh Evans
So… if my generic’s Cmax is 118% of the brand, I’m good? No worries? Cool. I’ll take it.
Allison Reed
Thank you for writing this with so much clarity. It’s rare to see complex science explained without jargon-and even rarer to feel hopeful about the system afterward. This is the kind of post that reminds me why I believe in evidence-based medicine.