When your immune system turns on your own body, things get messy fast. Autoimmune diseases like lupus, rheumatoid arthritis, and vasculitis cause swelling, pain, and organ damage because your body’s defenses go rogue. That’s where corticosteroids come in - fast, powerful, and often life-saving. But they’re not a magic fix. They work wonders in the short term, but if you’re on them for months or years, the price can be steep.
How Corticosteroids Quiet Down Autoimmune Attacks
Corticosteroids, like prednisone and methylprednisolone, are synthetic versions of cortisol, the hormone your adrenal glands make when you’re stressed. They don’t cure autoimmune diseases. Instead, they slam the brakes on inflammation - the main driver of damage in these conditions.
These drugs bind to receptors inside nearly every cell in your body. Once attached, they switch off genes that crank out inflammatory proteins like tumor necrosis factor and interleukins. They also block enzymes like phospholipase A2, which produce chemicals that attract immune cells to inflamed areas. The result? Swelling drops, pain fades, and organs stop being attacked.
Unlike drugs like methotrexate or azathioprine, which take weeks to kick in, corticosteroids work in hours. That’s why they’re the first line of defense in flare-ups - whether it’s sudden kidney damage from lupus, a severe asthma attack, or a rash that won’t quit. For aggressive diseases like Wegener’s granulomatosis or Goodpasture’s syndrome, doctors often use high-dose IV methylprednisolone pulses to stop damage before it’s irreversible.
When Corticosteroids Work - and When They Don’t
They’re not a one-size-fits-all solution. Corticosteroids shine in diseases where inflammation is the main problem: systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease, and vasculitis. In early-stage type 1 diabetes or primary biliary cholangitis, where some insulin-producing or bile duct cells are still alive, they can help slow damage. But once those cells are gone, steroids won’t bring them back.
They’re useless in advanced cases of Hashimoto’s thyroiditis, Graves’ disease, and late-stage primary biliary cholangitis. Why? Because the problem isn’t inflammation anymore - it’s permanent tissue loss. No amount of steroid can rebuild a destroyed thyroid or liver duct. In multiple sclerosis, steroids help during acute relapses by calming nerve inflammation, but they don’t stop long-term degeneration.
Even in conditions where they help, doctors aim for the lowest possible dose. The goal isn’t just to control symptoms - it’s to get you off steroids entirely, or at least keep you on the smallest dose that still works. That’s why they’re often paired with other drugs like azathioprine, mycophenolate, or rituximab. These help maintain control so you don’t need to keep cranking up the steroid dose.
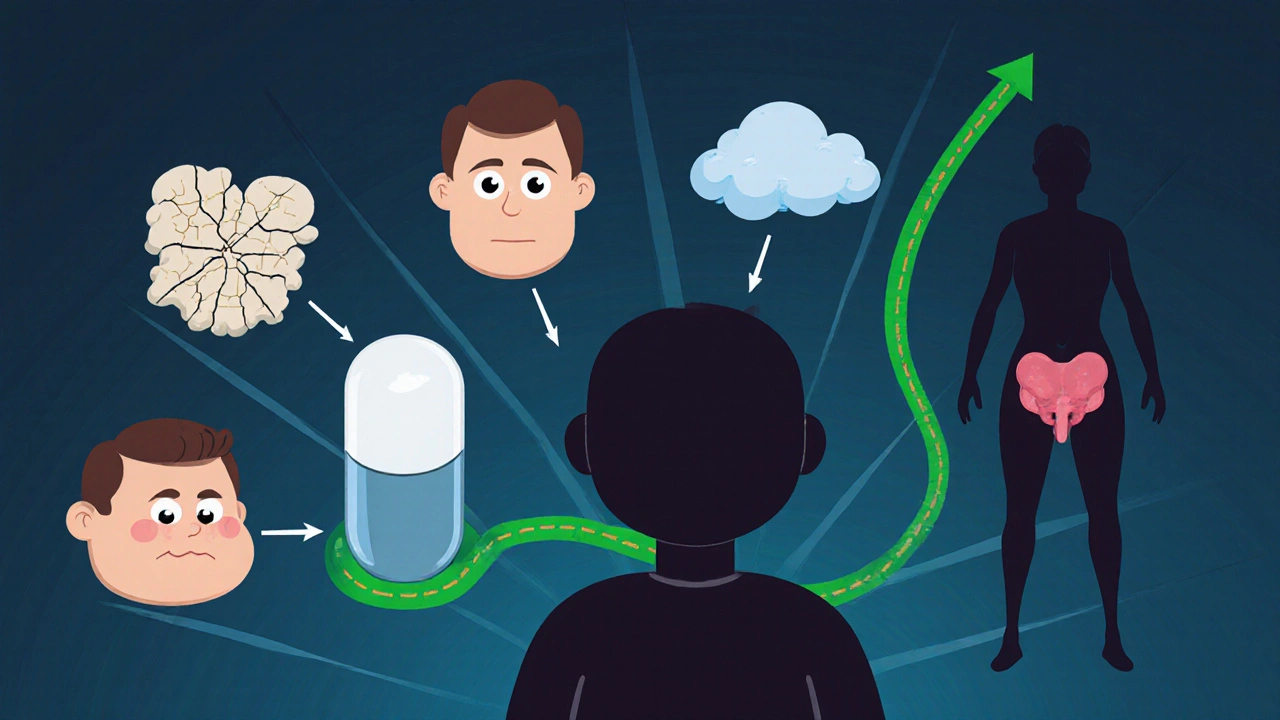
The Hidden Cost: Long-Term Side Effects
Here’s the catch: the longer you’re on corticosteroids, the more your body pays the price. The same mechanisms that calm your immune system also mess with bone, muscle, blood sugar, and your brain’s natural hormone balance.
Osteoporosis is one of the most serious risks. Steroids interfere with bone-building cells and increase calcium loss. After just three months on prednisone, bone density can drop significantly. That’s why anyone on steroids for more than three months gets a bone scan and is often prescribed calcium, vitamin D, and bisphosphonates to protect their skeleton.
Cataracts and glaucoma are common too. Steroids increase pressure inside the eye and cloud the lens. Regular eye checks are non-negotiable if you’re on long-term therapy.
Weight gain, especially around the face, belly, and back of the neck, is almost universal. It’s not just fat - it’s fluid retention and muscle breakdown. Many people gain 10-20 pounds without changing their diet. That’s why diet and light exercise are part of the treatment plan.
Steroids also mess with your blood sugar. Even people without diabetes can develop steroid-induced diabetes. Fasting blood sugar above 7.0 mmol/L on therapy is a red flag. Some patients end up on metformin or insulin just to manage this side effect.
And then there’s adrenal insufficiency. Your body stops making its own cortisol because it thinks the drug is doing the job. If you suddenly stop taking steroids, you can go into adrenal crisis - low blood pressure, vomiting, confusion, even death. That’s why tapering off slowly is critical. Doctors test for this by checking morning cortisol levels after skipping a dose for 24 hours.
Other side effects? Mood swings, trouble sleeping, thinning skin, easy bruising, and increased risk of infections. Even minor cuts can turn into serious wounds. Sun sensitivity is real too - your skin burns faster and can darken unevenly.
How to Use Them Safely - The Modern Approach
Today’s treatment isn’t about riding out the side effects. It’s about minimizing exposure. The Australian Prescriber and Johns Hopkins both emphasize: use the lowest effective dose for the shortest time possible.
For example, in rheumatoid arthritis, a short burst of prednisone (5-10 mg daily) might be used to control flares while waiting for methotrexate to work. Once the disease is stable, the steroid is slowly lowered and stopped. In lupus nephritis, a high-dose pulse might be given for three days, followed by a rapid taper to a low daily dose, then switched to a non-steroid immunosuppressant.
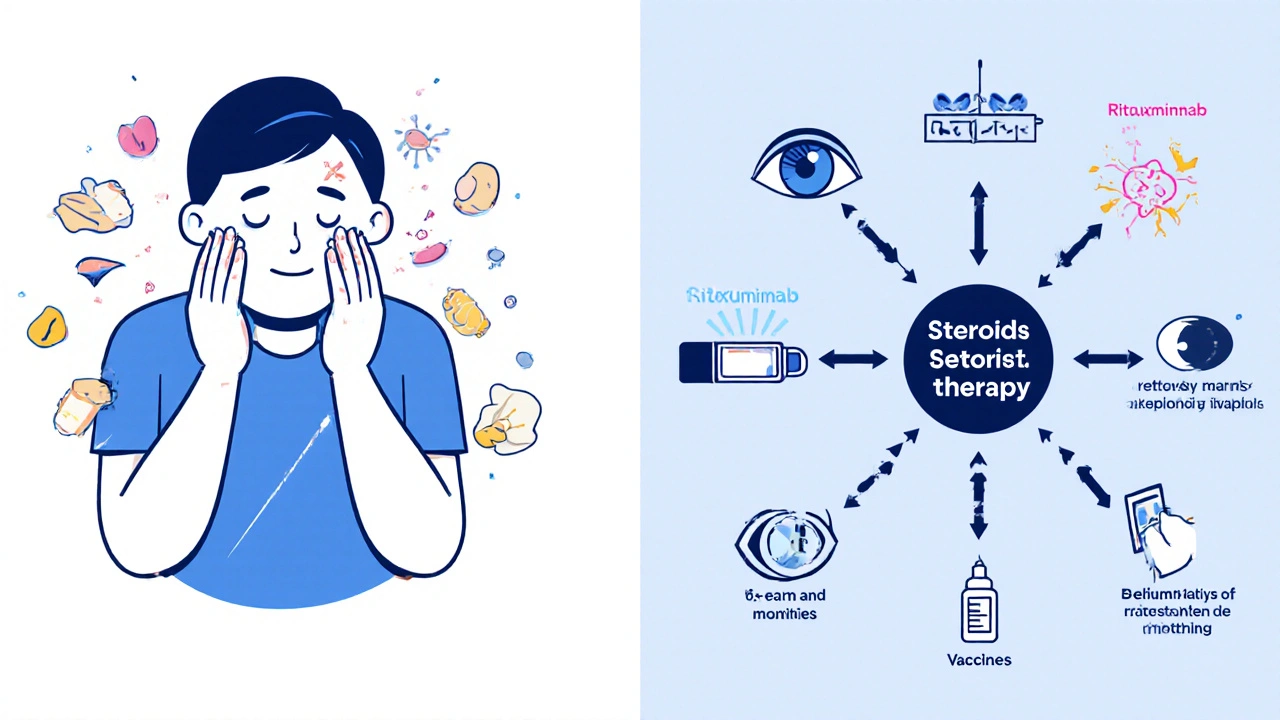
Topical steroids - creams, inhalers, nasal sprays - are preferred when possible. For asthma, inhaled corticosteroids deliver the drug straight to the lungs with almost no systemic effect. For eczema or psoriasis, topical creams reduce skin inflammation without wrecking your bones.
Combination therapy is now standard. Studies show that adding rituximab to prednisone in autoimmune hemolytic anemia cuts relapse rates in half. In severe lupus, combining steroids with belimumab or mycophenolate reduces steroid dependence by 40% over two years.
Even the timing matters. Taking your dose in the morning mimics your body’s natural cortisol rhythm and reduces the risk of adrenal suppression. Evening doses? They’re a no-go unless specifically needed.
What to Do If You’re on Long-Term Steroids
If you’ve been on corticosteroids for more than three months, you need a plan. Here’s what works:
- Get a bone density scan - every year if you’re on more than 5 mg of prednisone daily.
- Take 1,200 mg of calcium and 800-1,000 IU of vitamin D daily.
- See an eye doctor annually for glaucoma and cataract screening.
- Monitor your blood sugar - check fasting glucose every 3-6 months.
- Never stop cold turkey. Always taper under medical supervision.
- Get vaccinated. Flu, pneumonia, and shingles shots are essential - steroids weaken your defenses.
- Wear sunscreen daily. Even on cloudy days.
- Exercise. Weight-bearing activities like walking or resistance training help protect bone and muscle.
Some people worry about steroid dependence. But dependence isn’t addiction - it’s physiology. Your body forgot how to make cortisol. That’s fixable. With a slow, smart taper, most people regain normal adrenal function within months.
The Future: Less Steroid, More Precision
The future of autoimmune treatment isn’t more steroids - it’s smarter, targeted drugs. Biologics like rituximab, tocilizumab, and anifrolumab block specific parts of the immune system without wiping out the whole thing. They’re expensive, but they’re safer long-term.
Researchers are even looking at GILZ, a protein that helps corticosteroids do their anti-inflammatory job. If they can create a drug that mimics GILZ without the side effects, we might finally have a steroid alternative that’s both powerful and safe.
For now, corticosteroids remain the fastest, most reliable tool we have to stop autoimmune flare-ups in their tracks. But they’re a bridge - not a destination. The goal isn’t to live on them. It’s to use them to get to a place where you don’t need them anymore.
Can corticosteroids cure autoimmune diseases?
No, corticosteroids don’t cure autoimmune diseases. They control inflammation and suppress immune attacks, which reduces symptoms and prevents damage. But they don’t fix the underlying immune malfunction. Once you stop taking them, the disease can return. That’s why they’re used to buy time - while other drugs, like biologics or disease-modifying agents, take effect.
How long can you safely take prednisone?
There’s no fixed safe duration, but the risk of serious side effects rises sharply after three months. Doctors aim to keep patients on prednisone for under six months if possible. For chronic conditions, the goal is to taper to 5 mg daily or less within a year. Any use beyond a year requires careful monitoring for bone loss, eye problems, and adrenal suppression.
Do all corticosteroids have the same side effects?
The core side effects - bone loss, weight gain, high blood sugar, cataracts - are similar across all systemic corticosteroids like prednisone, methylprednisolone, and hydrocortisone. But potency varies. Methylprednisolone is about 5 times stronger than hydrocortisone, so lower doses are needed. Topical, inhaled, or injected versions have far fewer side effects because they don’t circulate widely in the blood.
Why do steroids cause weight gain?
Steroids increase appetite and cause your body to hold onto fluid. They also change how fat is stored - pushing it toward your belly, face, and upper back. At the same time, they break down muscle, especially in the arms and legs. This combination leads to rapid weight gain, even if you’re eating normally. It’s not laziness - it’s biology.
Can I stop taking steroids if I feel better?
Never stop abruptly. Even if you feel great, your adrenal glands may have shut down. Stopping suddenly can trigger adrenal crisis - low blood pressure, nausea, confusion, and collapse. Always taper slowly under your doctor’s guidance. A typical taper might drop the dose by 1-2.5 mg every 1-2 weeks, depending on how long you’ve been on it.
Are there natural alternatives to corticosteroids?
No natural supplement can match the power of corticosteroids to stop an autoimmune flare. Turmeric, fish oil, or vitamin D may help reduce mild inflammation, but they won’t control severe disease. Relying on them instead of prescribed steroids can lead to irreversible organ damage. Always talk to your doctor before changing treatment.
What’s the difference between corticosteroids and anabolic steroids?
They’re completely different. Corticosteroids reduce inflammation and suppress the immune system. Anabolic steroids mimic testosterone to build muscle. People confuse them because both have “steroid” in the name, but they act on different receptors and have opposite effects. Anabolic steroids are not used for autoimmune diseases.


8 Comments
Jeremy Hernandez
Look i get it steroids save lives but come on man they turn you into a puffed up zombie with diabetes and brittle bones. I know a guy who took prednisone for 2 years and ended up needing a hip replacement at 38. They’re not medicine they’re chemical handcuffs. And don’t even get me started on how Big Pharma pushes these like candy.
Tarryne Rolle
It’s ironic isn’t it? We poison our bodies to stop our bodies from poisoning themselves. The irony of modern medicine is that it treats symptoms like enemies instead of messages. Maybe the immune system isn’t broken… maybe it’s screaming because we’ve been ignoring it for decades. Sugar, stress, toxins - we’re the original autoimmune trigger.
Kyle Swatt
Man steroids are like a flamethrower in a library - you burn the fire down but everything else turns to ash. I’ve seen people come back from the brink with these things, yeah. But then they’re stuck in this limbo where their body forgets how to breathe without the crutch. It’s not just side effects - it’s identity loss. Who are you when your adrenal glands don’t know their job anymore? You’re not sick. You’re a ghost of your biology.
And don’t even get me started on how we treat chronic illness like a temporary glitch instead of a whole new operating system. We don’t adapt. We just keep throwing bigger bombs at the problem.
Deb McLachlin
The clinical guidelines outlined in this post are comprehensive and align with current best practices in rheumatology and immunology. The emphasis on tapering protocols, bone density monitoring, and combination therapy with disease-modifying agents is evidence-based and reflects consensus recommendations from the American College of Rheumatology and the European League Against Rheumatism. It is also noteworthy that the distinction between systemic and localized corticosteroid administration is accurately presented, as this has direct implications for risk-benefit ratios in long-term management.
saurabh lamba
bro why even use steroids at all? just eat turmeric and chill. i mean look at yoga gurus in india they never take drugs and still live to 100. maybe your body just needs more sunlight and less stress? 🙏
Kiran Mandavkar
How can you even call this a medical breakthrough? This is just pharmaceutical slavery dressed up as science. You’re not treating disease - you’re trading one disaster for ten others. And you call this progress? The fact that we still rely on 1950s-era synthetic cortisol analogs while ignoring epigenetics, gut microbiome modulation, and metabolic reprogramming shows how bankrupt modern medicine really is. We’re patching a sinking ship with duct tape and calling it innovation.
Eric Healy
u gotta be kidding me they say dont stop cold turkey but nobody tells u how slow to taper. i was on 10mg for 8 months and doc said just drop by 1mg every 2 weeks. i did and nearly died. my bp dropped so hard i blacked out in the grocery store. like wtf. u need a damn map for this shit.
Shannon Hale
OMG YES. I’ve been on steroids for 5 years and I swear my skin looks like crepe paper. I bruise if someone sneezes near me. And the moon face? I stopped taking selfies. I look like a confused raccoon who ate a whole bag of chips. And don’t even get me started on the insomnia and rage. I yelled at my cat for 20 minutes because she looked at me wrong. She’s fine. I’m not. This isn’t medicine - it’s a slow-motion horror movie and the studio won’t let me quit.