When you pick up a prescription, you might see a label that says generic instead of the brand name you recognize. It’s cheaper. It’s widely used. But do you worry it might cause more side effects? You’re not alone. Many patients report feeling worse after switching from a brand-name drug to its generic version. Some even swear their anxiety, fatigue, or headaches got worse overnight. So is this just in their head-or is there real risk?
What Does "Bioequivalent" Actually Mean?
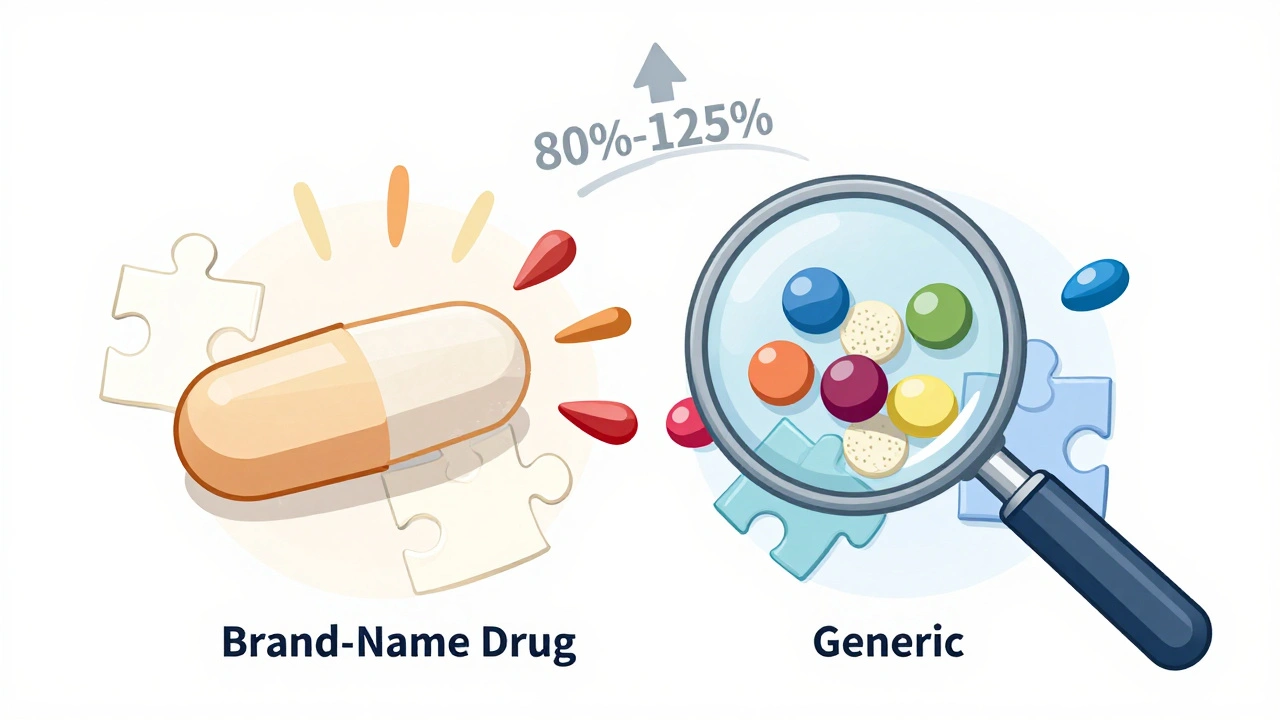
The FDA says generics are just as safe and effective as brand-name drugs. That’s because they must be bioequivalent. That means the active ingredient-the part that actually treats your condition-must enter your bloodstream at the same rate and in the same amount as the brand-name version. The acceptable range? Between 80% and 125% of the brand’s absorption rate. Sounds strict, right? But here’s the catch: that 45% window is wide. For most drugs, it doesn’t matter. Your body can handle small swings in how fast or how much gets absorbed. But for drugs with a narrow therapeutic index-like warfarin, levothyroxine, or phenytoin-that small difference can be meaningful. A 10% drop in absorption might mean your blood clotting time goes off. A 15% spike could trigger seizures. That’s why doctors sometimes ask pharmacists to "dispense as written" for these meds: to keep you on the same manufacturer’s version.Do Generics Cause More Side Effects? The Data Says Mostly No
A major 2018 study in PLOS Medicine looked at over 38 clinical trials involving heart drugs, thyroid meds, diabetes pills, and antidepressants. The result? No meaningful difference in hospitalizations, heart attacks, or death rates between brand and generic users. The same held true for drugs like alendronate, glipizide, and quinapril. Other large studies confirmed this: for the vast majority of patients, generics work just as well and are just as safe. Even when researchers dug into adverse event reports from the FDA’s database, they found something surprising. Authorized generics-those made by the original brand company under a different label-had far fewer reports than regular generics. That suggests it’s not the active ingredient causing the problem. It’s something else.The Real Culprit: Inactive Ingredients and the Nocebo Effect
Generics don’t have to match the brand’s fillers. That means different dyes, binders, coatings, or preservatives. If you’re sensitive to lactose, gluten, or certain food dyes, switching to a generic might trigger a reaction-not because the medicine doesn’t work, but because your body reacts to the new filler. Then there’s the nocebo effect. That’s the opposite of placebo. If you believe generics are inferior, your brain can start producing symptoms to match that belief. One study gave people identical placebo pills but told half they were brand-name and half they were generic. The "generic" group reported significantly more side effects-even though the pills were the same. Another study found patients were more likely to quit taking a pill if they thought it was generic. In one 7-day trial, 54% of people stopped taking a placebo labeled as generic, compared to just 33% who thought it was brand-name. That’s not a drug effect. That’s a perception effect.
Why Do Some People Report Worse Reactions?
Let’s be honest: some people do feel worse after switching. And it’s not all in their heads. Take levothyroxine. Many patients report mood swings, weight gain, or heart palpitations after switching from Synthroid to a generic. Why? Because even tiny changes in thyroid hormone levels can ripple through your whole system. The FDA allows up to 5% variation in potency between batches of the same generic. For a thyroid patient, that’s enough to feel off. Same with bupropion. Patients switching from Wellbutrin XL to generic versions have reported increased anxiety, insomnia, and irritability. The active ingredient is identical. But the extended-release coating? That’s different. It changes how fast the drug releases. Some people’s bodies don’t adjust well. And then there’s the manufacturing issue. A 2022 Ohio State University study found that generics made in India were linked to 54% more severe adverse events-hospitalizations, disability, even deaths-than those made in the U.S. The researchers pointed to mature generics (drugs that’ve been on the market for years) as the main problem. That suggests quality control can slip over time, especially with high-volume, low-margin production.What About the Numbers? Real-World Data
Let’s look at some hard stats:- For simvastatin, patients on the brand version were 80% more likely to quit taking it than those on the generic. The generic was better tolerated.
- For losartan, emergency room visits jumped 8% after generics hit the market. But was that because the drug was worse-or because more people started taking it because it was cheaper?
- In one analysis of 136,000 older adults on blood pressure meds, hospitalizations rose after generics became available. But again, no proof the drug caused it.
What Should You Do If You Think Your Generic Isn’t Working?
If you switch to a generic and feel worse, don’t assume it’s your fault. Don’t assume it’s all in your head. Do this:- Track your symptoms. Write down what changed-sleep, mood, energy, pain levels-before and after the switch. Be specific.
- Check the pill. Look at the imprint code on the pill. If it’s different from your old one, you switched manufacturers. Try going back to the original generic version.
- Talk to your pharmacist. Ask if the generic you got is made by the same company as before. Ask if there’s a different generic version available.
- Ask your doctor. If you’re on a narrow-therapeutic-index drug, ask them to write "Dispense as Written" on your prescription. It may cost more, but it keeps you on the same formulation.
- Report it. If you have a serious reaction, report it to the FDA’s MedWatch program. Your report helps spot patterns.
The Bottom Line: Generics Are Safe-But Not Always Identical
For 9 out of 10 people, generics are just as safe and effective as brand-name drugs. They save billions of dollars every year. They’re not dangerous. They’re not scams. But they’re not always identical. The active ingredient? Same. The fillers? Sometimes different. The release mechanism? Often different. The manufacturing quality? Sometimes inconsistent, especially overseas. If you’re on a critical medication-thyroid, seizure, blood thinner-stick with the same manufacturer if you can. If you feel off after switching, speak up. Your experience matters. The science says most people are fine. But medicine isn’t one-size-fits-all. Your body might react differently. And that’s okay. The goal isn’t to scare you away from generics. It’s to help you understand when to pay attention-and when to trust the system.Are generic drugs less safe than brand-name drugs?
For most people, no. The FDA requires generics to meet the same strict standards for quality, strength, and purity as brand-name drugs. Large studies show no significant difference in safety or effectiveness for the vast majority of medications. However, rare cases exist where differences in inactive ingredients or manufacturing quality may cause reactions in sensitive individuals.
Why do some people feel worse after switching to a generic?
There are two main reasons. First, generics can use different fillers, dyes, or coatings, which might trigger allergies or sensitivities. Second, the "nocebo effect"-where expecting side effects causes you to experience them-plays a big role. Studies show people report more side effects when they believe they’re taking a generic, even if the pill is identical to the brand.
Which medications should I avoid switching to generics?
For drugs with a narrow therapeutic index-where small changes in dosage can cause big effects-doctors often recommend staying on the same version. These include levothyroxine (for thyroid), warfarin (for blood thinning), phenytoin (for seizures), and some antidepressants like bupropion. If you’re on one of these, ask your doctor to write "Dispense as Written" on your prescription.
Are generics made in India less safe?
Some studies suggest that generics manufactured in India are linked to higher rates of severe adverse events compared to those made in the U.S., particularly for older, mature generic drugs. However, the FDA disputes that location alone determines safety, noting that many U.S.-made generics also come from overseas facilities. The key issue is consistent quality control, not geography.
Can I ask my pharmacist to give me the brand name instead?
Yes, but it may cost more. Your pharmacist is required to give you the generic unless your doctor writes "Dispense as Written" on the prescription or you specifically request the brand. Some insurance plans won’t cover the brand unless the generic fails. If you’re experiencing side effects, ask your doctor to write "Do Not Substitute"-that ensures you get the version that works for you.
How can I tell if my generic is from a different manufacturer?
Check the shape, color, and imprint code on the pill. If it looks different from your last refill, it’s likely a different manufacturer. You can also ask your pharmacist which company made it. If you’ve had issues before with a certain version, ask to stick with the one that worked.


10 Comments
Olivia Portier
So I switched my thyroid med to generic last year and thought I was gonna die. Sweating at 3am, heart racing, felt like a zombie. Turns out it was a different filler-lactose. I’m lactose intolerant and didn’t even think about it. My pharmacist was like ‘oh yeah, this batch has it’ and switched me back. Now I check the pill imprint like a hawk. Don’t assume it’s all in your head-sometimes it’s just the dye.
Tiffany Sowby
Of course generics are fine. If you can’t afford the brand, you’re just weak. America makes the best medicine. If you feel weird, maybe you’re just lazy and not taking it right. Stop whining.
Asset Finance Komrade
One might argue that the very notion of ‘bioequivalence’ is a modern myth-a statistical illusion constructed by regulatory bodies to appease capitalist imperatives. The body is not a beaker. It is a symphony of chaos. A 5% variance in levothyroxine may be mathematically negligible, yet ontologically catastrophic. We reduce life to numbers, then wonder why we feel hollow. 🤔
Jennifer Blandford
OMG YES. I switched from Wellbutrin XL to generic and went from chill queen to screaming into a pillow at 2am. My therapist was like ‘did you change meds?’ and I was like ‘I didn’t even know I could!’ Then I found out the generic had a different coating. Now I pay extra to keep the same one. My mental health > my wallet. 💅
Brianna Black
As a clinical pharmacist with over 15 years in community practice, I can confirm that the variance in inactive ingredients is often the culprit-not the active pharmaceutical ingredient. Patients with known allergies to dyes or fillers are at disproportionate risk. Moreover, the nocebo effect is empirically validated and statistically significant in multiple double-blind studies. Education and pharmacist consultation are critical. Always verify the manufacturer code on the pill.
Stacy Tolbert
I took a generic blood thinner and ended up in the ER. My INR went through the roof. They said it was ‘within range’ but I bled for three days. My doctor told me to ‘tough it out’-like I was some kind of robot. I switched back to brand and my life returned. Don’t let them tell you it’s ‘all in your head’ when your body’s screaming.
Ryan Brady
India makes trash meds. That’s why. No debate. We should ban all foreign generics. America First. If you want safe medicine, pay for the brand. Period. 🇺🇸
Raja Herbal
Bro, I make generics in India. We follow FDA guidelines. But sometimes the machines are old, the power flickers, and someone forgets to label the batch. Not because we’re evil-because we’re under pressure to make 10 million pills a day for $0.02 each. You want safe? Pay more. Or learn to read the pill imprint. It’s not magic, it’s math.
Iris Carmen
i switched to generic zoloft and started crying at the grocery store for no reason. thought i was going crazy. turned out the new version had a different filler. my pharmacist gave me the old one back and i felt like a human again. soooo… yeah. check your pills. 🙃
Delaine Kiara
Let’s be real-this whole debate is a distraction. The real issue is that Big Pharma invented the brand-name myth to keep prices inflated. Generics are cheaper because they don’t spend $2 billion on ads and celebrity endorsements. The fact that some people feel worse? That’s because they were conditioned to believe brand = better. The nocebo effect is the real villain here. Also, if you’re on warfarin, yes, stick with one version. But for 90% of drugs? Chill. Your body doesn’t care if it’s called ‘Lipitor’ or ‘simvastatin’. It just wants to work. And if you’re still scared? Fine. Pay extra. But stop acting like you’re a medical genius because you read one Reddit thread.