AUC Explained: What It Means for Generic Drugs and Patient Safety
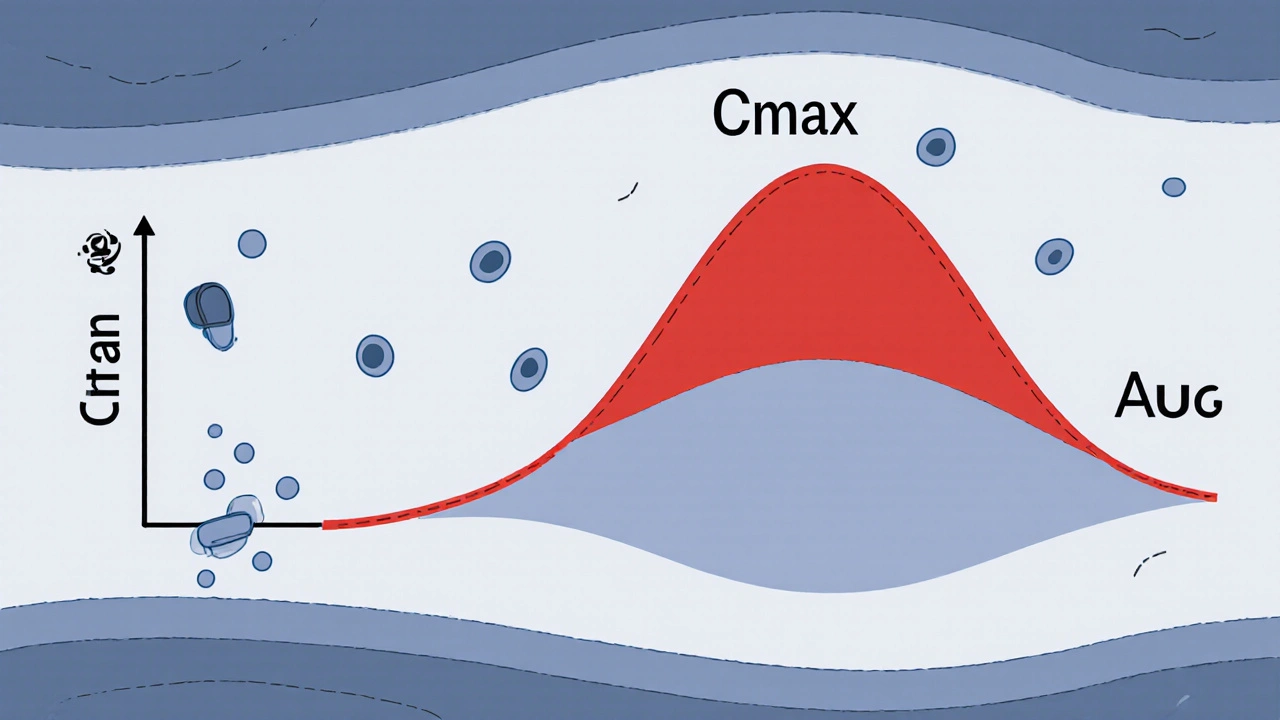
When you take a generic drug, you want to know it works just like the brand-name version. That’s where AUC, Area Under the Curve, a pharmacokinetic measure that tracks how much of a drug enters your bloodstream over time. Also known as area under the concentration-time curve, it’s one of the two main numbers regulators use to prove generics are safe and effective. If the AUC of a generic matches the brand drug within strict limits, it means your body absorbs it the same way—no surprises, no dangerous dips or spikes.
AUC doesn’t work alone. It’s paired with Cmax, the highest concentration of a drug in your blood after taking it, to give a full picture of how a drug behaves. Together, these two values tell regulators whether a generic will deliver the same results as the original. For drugs with a narrow therapeutic index—like warfarin or lithium—even a small difference in AUC can mean the difference between treatment and toxicity. That’s why the FDA, EMA, and Health Canada require generics to stay within 80% to 125% of the brand’s AUC to get approved.
These aren’t just lab numbers. They’re tied to real outcomes. A study published in the Journal of Clinical Pharmacology found that patients switching to generics with mismatched AUC had higher rates of hospital visits due to unstable blood levels. That’s why bioequivalence studies are done with healthy volunteers, using blood samples taken over hours after dosing. The data gets plotted into a curve—the AUC—and compared side-by-side with the brand. If the curves overlap closely enough, the generic gets the green light.
But AUC isn’t just about approval. It’s about trust. When pharmacists see a generic with proven AUC equivalence, they can confidently dispense it without worrying about hidden risks. When patients take their medication, they can be sure the dose they’re getting will work as expected. That’s the whole point: safety, consistency, and cost savings without compromise.
Below, you’ll find real-world posts that dig into how AUC shapes drug safety, why some medications need tighter rules, and how bioequivalence studies keep patients protected. Whether you’re a healthcare worker, a patient on generics, or just curious about how meds are tested, these articles break down the science without the jargon.