Biologic Drugs: What They Are, How They Work, and Why They Matter
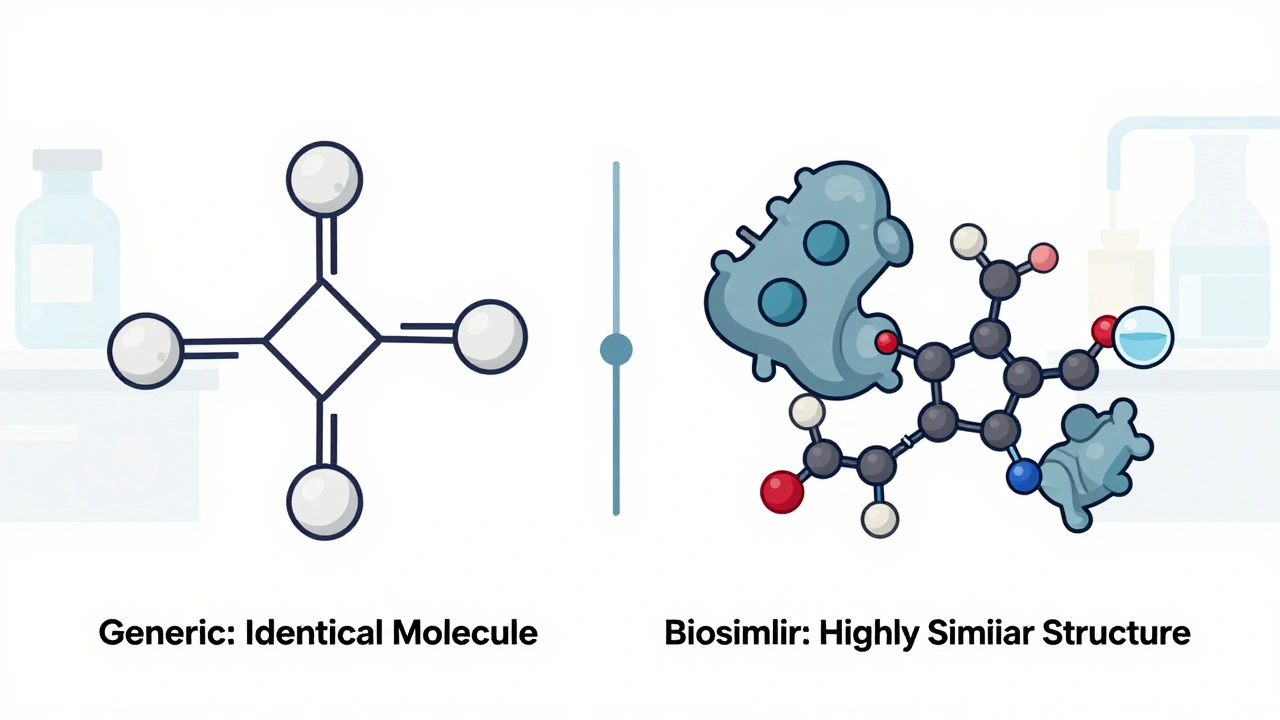
When you hear biologic drugs, medications made from living organisms like cells or proteins, used to treat chronic and life-threatening conditions. Also known as biologics, they are not like traditional pills you swallow. These drugs are grown in labs using living cells—often from bacteria, yeast, or animal tissue—and are designed to target very specific parts of your immune system or disease process. Unlike chemical-based drugs, biologics are huge, complex molecules that can’t be easily copied. That’s why you can’t just buy a generic version of Humira or Enbrel—they’re too intricate to replicate exactly.
This complexity leads to something called biosimilars, highly similar versions of biologic drugs approved after the original patent expires. Also known as follow-on biologics, they’re not identical to the original, but they’re close enough to work the same way in most patients. The FDA and EMA require strict testing to prove biosimilars match the original in safety and effectiveness. You’ll see this in posts about bioequivalence, the process of proving two drugs behave the same way in the body. Also known as pharmacokinetic similarity, it’s a big deal for biologics because even tiny differences can affect how well they work or whether they cause side effects. That’s why bioequivalence rules for biologics are stricter than for regular generics. For example, a generic version of aspirin just needs to release the same amount of active ingredient. But a biosimilar to a biologic drug must match in structure, how it’s absorbed, how long it lasts in your body, and even how your immune system reacts to it.
Biologic drugs are mostly used for conditions that don’t respond well to traditional treatments: rheumatoid arthritis, Crohn’s disease, psoriasis, certain cancers, and multiple sclerosis. They’re often given by injection or infusion because your stomach would break them down if you took them as a pill. They’re also expensive—sometimes tens of thousands a year—which is why many people look for alternatives. That’s where biosimilars come in. They’re cheaper, but still require careful monitoring. Some patients report different side effects with biosimilars, not because they’re unsafe, but because the immune system can be picky about tiny changes in the molecule. That’s why pharmacovigilance and adverse event reporting are so important in this space.
You’ll find posts here that dig into how these drugs are tested, why some people react differently, and how pharmacogenetic testing might one day help match the right biologic to the right patient. There are also discussions on how to spot counterfeit versions online, what to expect when switching from a brand-name biologic to a biosimilar, and how insurance and pricing affect access. Whether you’re a patient, caregiver, or just curious, understanding biologic drugs means understanding the future of medicine—more targeted, more powerful, but also more complex.