Biosimilar vs Generic: What’s the Real Difference?
When you hear biosimilar, a type of medication designed to be highly similar to an existing biologic drug, with no clinically meaningful differences in safety or effectiveness. Also known as follow-on biologics, it is not the same as a generic drug, even though both aim to lower costs. Most people think if it’s cheaper and works like the brand name, it’s just a copy. But with biologics, complex medicines made from living cells, like insulin, rheumatoid arthritis treatments, or cancer therapies, that’s not true. You can’t just reverse-engineer them like you can with a pill made of simple chemicals.
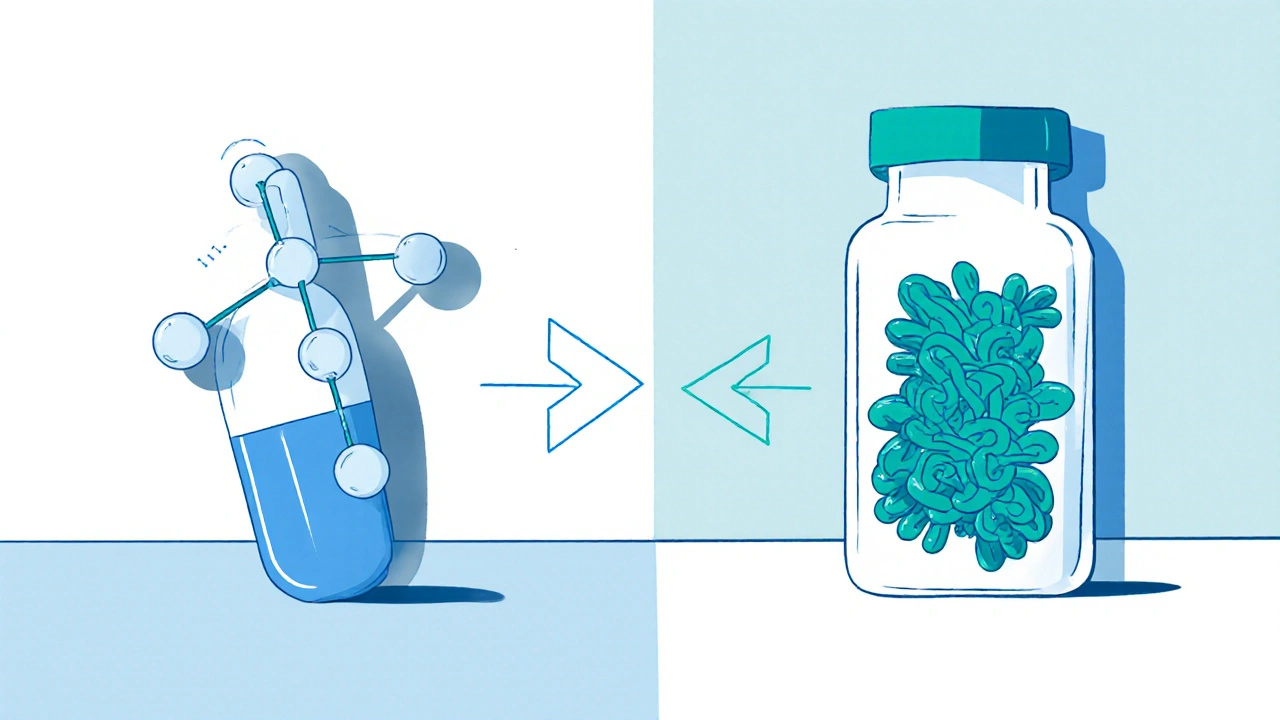
Generic drugs, chemically identical copies of brand-name pills, approved based on bioequivalence studies are straightforward. If the active ingredient matches, the dose is the same, and it works the same way — it gets approved. But biosimilars, are not exact copies — they’re highly similar versions of complex biologic drugs, made using living cells that can’t be perfectly replicated. Even tiny changes in how they’re made — the cell line, the temperature, the purification process — can affect how they behave in your body. That’s why biosimilars need way more testing than generics. They’re not just chemically checked; they’re tested in clinical trials to prove they work the same way and don’t cause more side effects.
Why does this matter? If you’re on a biologic like Humira or Enbrel for arthritis, switching to a biosimilar might save you hundreds a month. But if you’re on a simple pill like metformin or lisinopril, the generic version is just as safe and effective — and often cheaper. Insurance companies push biosimilars because they’re cheaper than the original biologic, but not always cheaper than a generic. And while generics have been around for decades, biosimilars are still new in many places, so not every pharmacy stocks them.
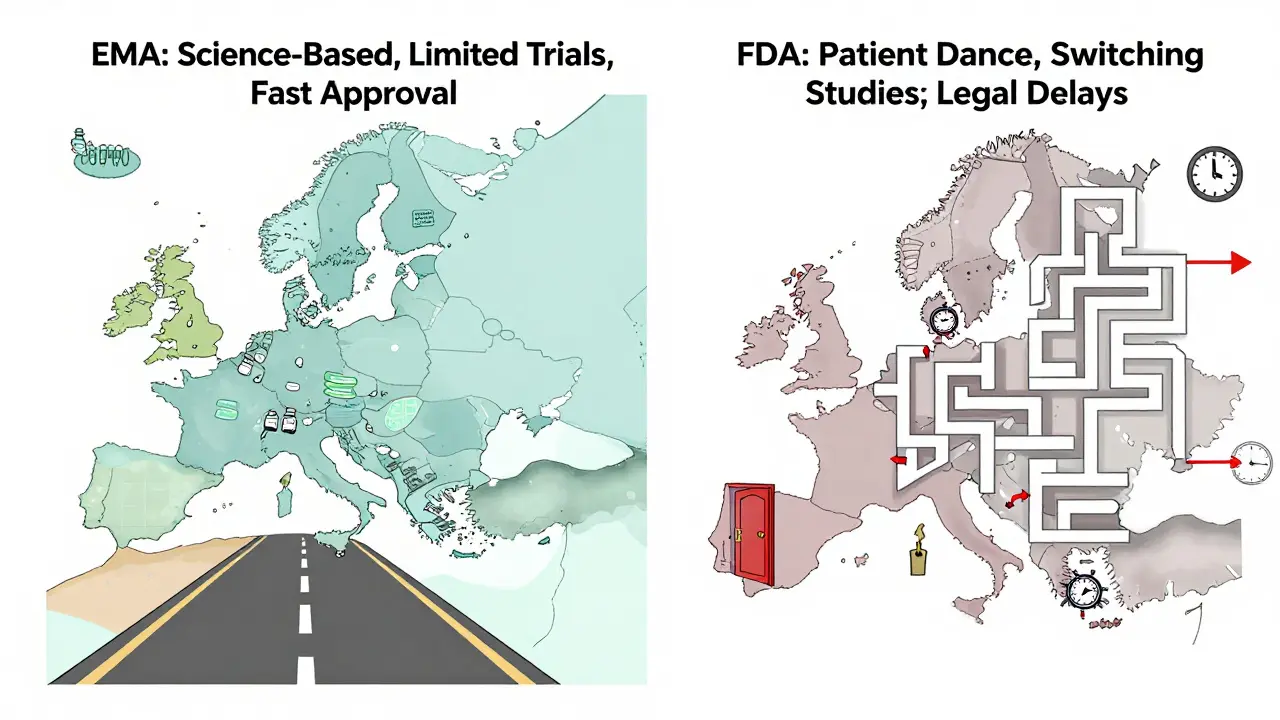
Some people worry biosimilars are riskier. They’re not. The FDA and other global regulators approve them only after proving they’re as safe and effective as the original. But because biologics are so complex, they can trigger different immune responses in some people. That’s why doctors often monitor you more closely when switching. You won’t get a biosimilar unless your doctor says it’s right for you.
What you’ll find in the posts below are real comparisons — from how biosimilar and generic drugs stack up in cost, to how they’re regulated, who prescribes them, and which ones actually save you money. You’ll see how drugs like insulin, rheumatoid arthritis treatments, and even cancer meds fit into this picture. There’s no marketing fluff — just clear facts on what works, what doesn’t, and what you should ask your pharmacist or doctor before making a switch.