Difference Between Biosimilars and Generics: What You Need to Know
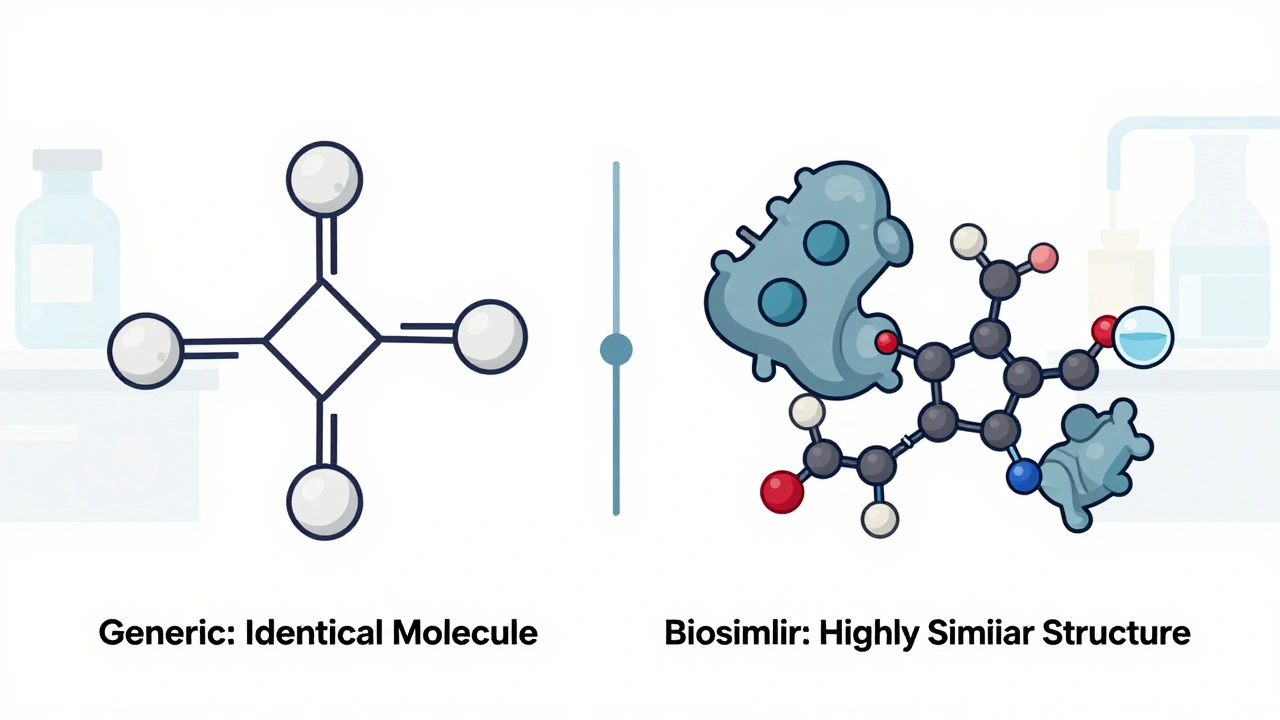
When you hear biosimilars, highly similar versions of complex biologic drugs made from living cells and generics, exact chemical copies of simple, small-molecule drugs, it’s easy to think they’re just two names for the same thing. But they’re not. Biologic drugs, medications made from living organisms like proteins or antibodies—used for cancer, autoimmune diseases, and rare conditions—are too complex to copy exactly. That’s why biosimilars aren’t called generics. They’re highly similar, but not identical. And that tiny difference changes how they’re tested, approved, and even prescribed.
Generics, on the other hand, are straightforward. If your doctor prescribes ibuprofen or metformin, the pharmacy can hand you a generic version that has the exact same active ingredient, dose, and effect as the brand name. The FDA requires generics to match the original in strength, purity, and how fast they’re absorbed. That’s why you can swap them without worry. But biosimilars? They’re made from living cells—think yeast or hamster ovaries—so tiny changes in the process can change how the drug works. That’s why they need dozens of tests: protein structure, immune response, stability, and even how they bind to targets in your body. The FDA and EMA don’t just check if they work—they check how they work at a molecular level.
Here’s what it means for you: if you’re on insulin, rheumatoid arthritis meds like Humira, or a cancer drug like Herceptin, switching to a biosimilar isn’t like switching from brand-name Advil to store-brand ibuprofen. Your doctor might need to explain why a biosimilar is safe for you, and you might need to monitor for subtle changes. But the upside? Biosimilars cut costs by 15–35%, making life-saving treatments more affordable. Generics save even more—often 80% off—but only for simple chemical drugs. You can’t make a generic version of a biologic because it’s not just a molecule—it’s a living system. That’s why biosimilars exist: to bring down prices without sacrificing safety for complex conditions.
Some people worry that biosimilars are riskier. But data from the U.S., EU, and Canada show they’re just as safe as the originals when used correctly. The key is knowing which drug you’re getting. If your prescription says "adalimumab," it could be the brand or a biosimilar—both work, but you should be informed. Meanwhile, generics for things like lisinopril or atorvastatin? You can swap them freely. The real difference isn’t just science—it’s transparency. If you’re on a biologic, ask if a biosimilar is an option. If you’re on a simple pill, know your generic is just as good. This collection of posts dives into exactly how these drugs are tested, why some people notice differences, how pharmacists track safety, and what the latest rules mean for your health. You’ll find real stories, hard data, and clear explanations—no jargon, no fluff, just what you need to make smarter choices.