Drug Safety: What You Need to Know About Medication Risks and Protection
When you swallow a pill, you trust it will help—not hurt. That trust is built on drug safety, the system of rules, testing, and monitoring that ensures medications work as intended without causing avoidable harm. Also known as medication safety, it’s not just about the drug itself, but how it’s made, labeled, tracked, and used in real life. This system doesn’t work in a vacuum. It relies on data from patients, pharmacists, regulators, and labs working together to catch problems before they spread.
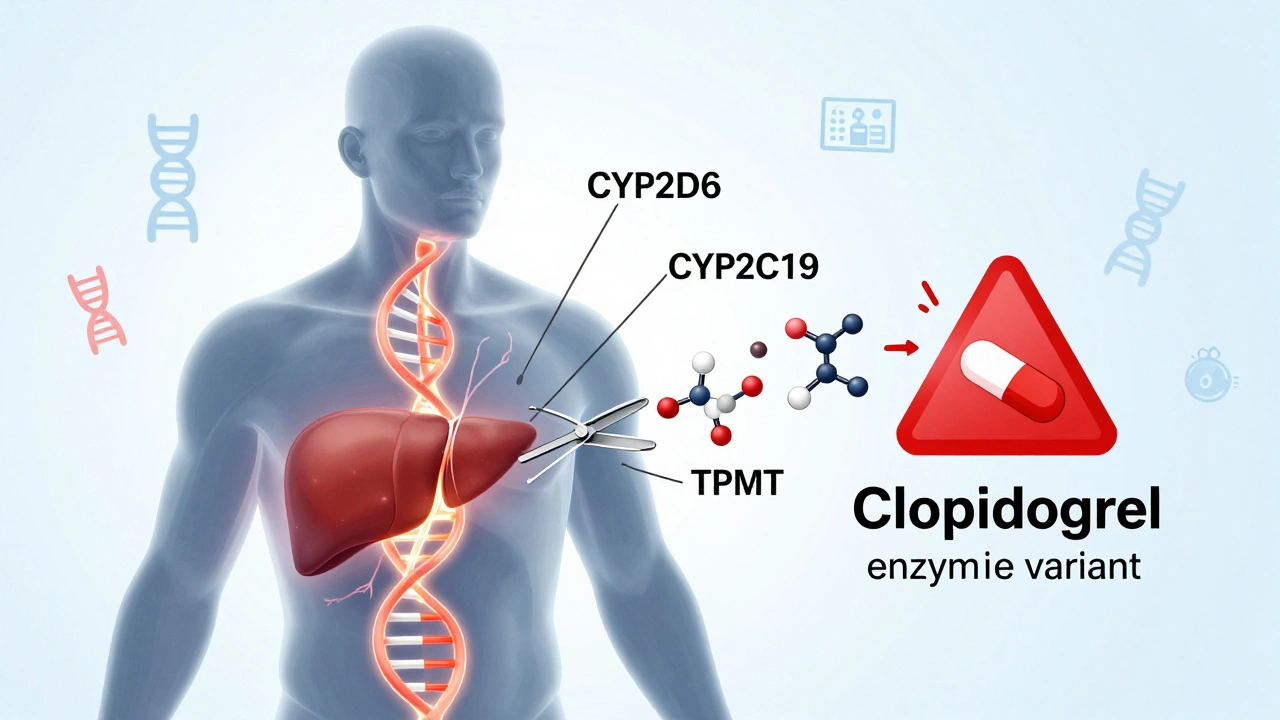
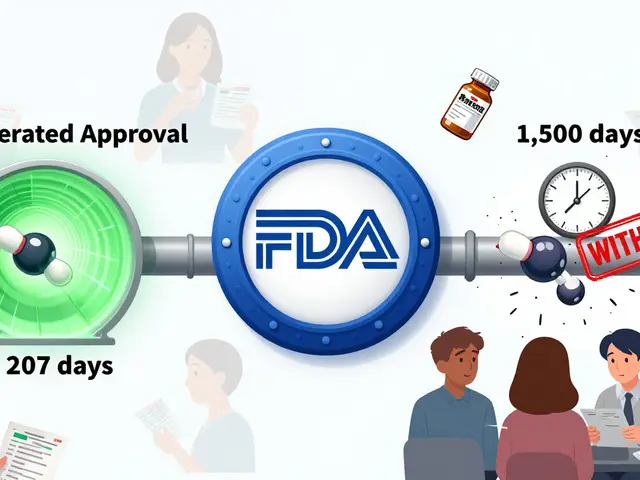
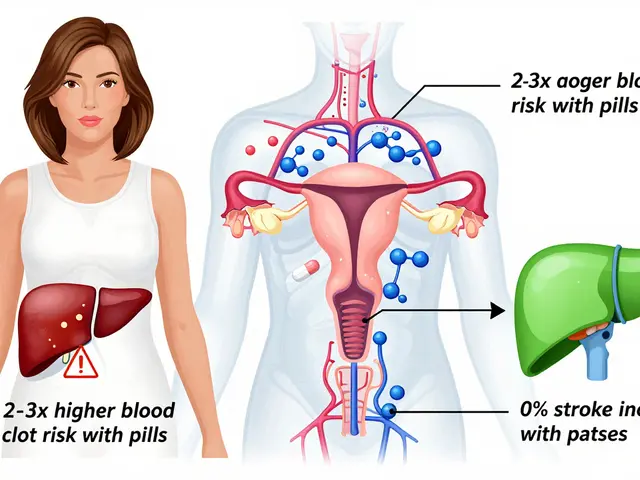
One of the most serious signals in drug safety is the boxed warning, the FDA’s strongest alert for life-threatening risks like liver failure, suicidal thoughts, or dangerous drug interactions. Also called black box warning, these aren’t just footnotes—they’re bold, mandatory labels that change how doctors prescribe and how patients think about their meds. Then there’s bioequivalence requirements, the strict tests that prove generic drugs behave the same as brand-name ones in your body. Without these, a cheaper version could be too weak, too strong, or cause unexpected side effects—especially with narrow therapeutic index drugs, where the difference between a cure and a crisis is tiny. And when something goes wrong? That’s where adverse event reporting, the process of doctors, pharmacists, and patients flagging unexpected reactions to medications, comes in. ADR reporting isn’t bureaucracy—it’s the early warning system that catches dangers no lab test ever could. These pieces—warnings, testing, reporting—are the backbone of keeping millions safe every day.
You don’t need to be a doctor to understand drug safety. You just need to know what to look for. A new label. A strange reaction. A generic that doesn’t feel right. These aren’t just inconveniences—they’re clues. The posts below show you exactly how drug safety works in practice: from how the FDA tracks life-threatening changes to why your pharmacist asks if you’re taking supplements before filling a script. You’ll see how a single wrong interaction can turn a common pill into a danger, how bioequivalence keeps generics reliable, and how reporting by real people stops tragedies before they multiply. This isn’t theory. It’s what keeps your medicine from becoming your next emergency.