Generics: What They Are, How They Work, and Why They Matter
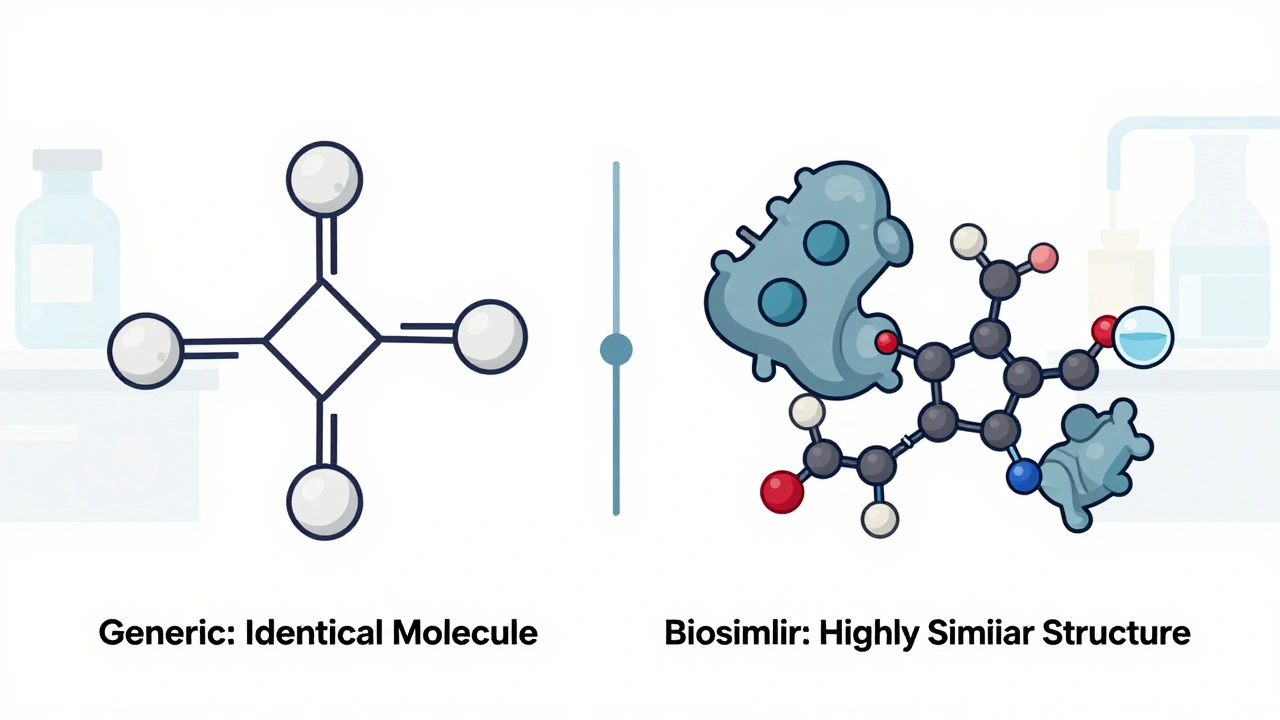
When you hear generics, copycat versions of brand-name drugs that contain the same active ingredient, dose, and intended use. Also known as generic medications, they're the reason millions can afford insulin, blood pressure pills, and antidepressants every month. They aren’t cheaper because they’re weaker—they’re cheaper because the patent ran out and other companies can make the same medicine without repeating the billion-dollar research.
But here’s what most people don’t realize: bioequivalence, the scientific process that proves a generic works just like the brand-name version isn’t just paperwork. It’s lab tests measuring how fast and how much of the drug enters your bloodstream—tracked by two numbers: Cmax, the highest concentration the drug reaches in your blood, and AUC, the total amount of drug your body is exposed to over time. If those numbers fall outside strict limits, the generic doesn’t get approved. The FDA, EMA, and Health Canada all enforce these rules. For most drugs, generics are identical in effect. But for narrow therapeutic index drugs, medicines where even tiny changes in dose can cause harm or fail to work—like warfarin, lithium, or levothyroxine—the rules are even tighter. That’s why you’ll see more scrutiny on those.
Still, people report side effects with generics that they didn’t have with the brand. Is that real? Sometimes. It’s not always the active ingredient—it’s the fillers, dyes, or coatings. Your body might react to a different binder in the generic tablet. Or your brain might expect it to feel different, and that perception can change how you feel. That’s why pharmacists are trained to watch for unusual reactions and report them. It’s not about blaming generics—it’s about understanding that medicine isn’t one-size-fits-all. Your genes, your gut, your other meds—all of it plays a part. That’s why adverse event reporting, the system that lets doctors and pharmacists flag unexpected reactions to any drug matters so much. Every report helps improve safety for everyone.
Generics aren’t a compromise. They’re a breakthrough. They’ve made life-saving drugs accessible to people who would otherwise go without. But they’re not magic. You still need to know what you’re taking, how to check it, and when to speak up. Below, you’ll find real stories, science-backed answers, and practical checks—from how to spot a fake online pharmacy selling generic Lamictal, to why some people swear their generic blood pressure pill doesn’t work like the brand, to what the data really says about side effects. This isn’t marketing. It’s what happens when patients, pharmacists, and scientists start asking the right questions.