Adverse Drug Reactions: What They Are, Why They Matter, and How to Spot Them
When you take a medicine, you expect it to help—not hurt. But sometimes, even the right drug at the right dose causes unexpected harm. That’s an adverse drug reaction, an unintended and harmful response to a medication given at normal doses for prevention, diagnosis, or therapy. Also known as ADR, it’s not a mistake—it’s a biological response that can range from a rash to heart failure. These reactions aren’t rare. Studies show nearly 1 in 5 hospital admissions in the U.S. are linked to ADRs, and many go unreported because people think it’s just "side effects" they have to live with.
What makes ADRs dangerous isn’t just the reaction itself—it’s how often they’re missed. Pharmacovigilance, the science of detecting, assessing, understanding, and preventing adverse effects of medicines, is the system designed to catch these problems before they hurt more people. It’s not just for big pharma—it’s for you, your pharmacist, and your doctor. When a patient reports a strange reaction after starting a new generic drug, that’s not just a complaint. It’s data. That data feeds into adverse event reporting, the formal process where healthcare providers and patients submit safety concerns to regulatory agencies like the FDA. These reports are how we find out that a cheap generic version of a blood thinner might cause more bleeding than the brand name, or that a new antibiotic triggers a rare but deadly skin condition in certain people.
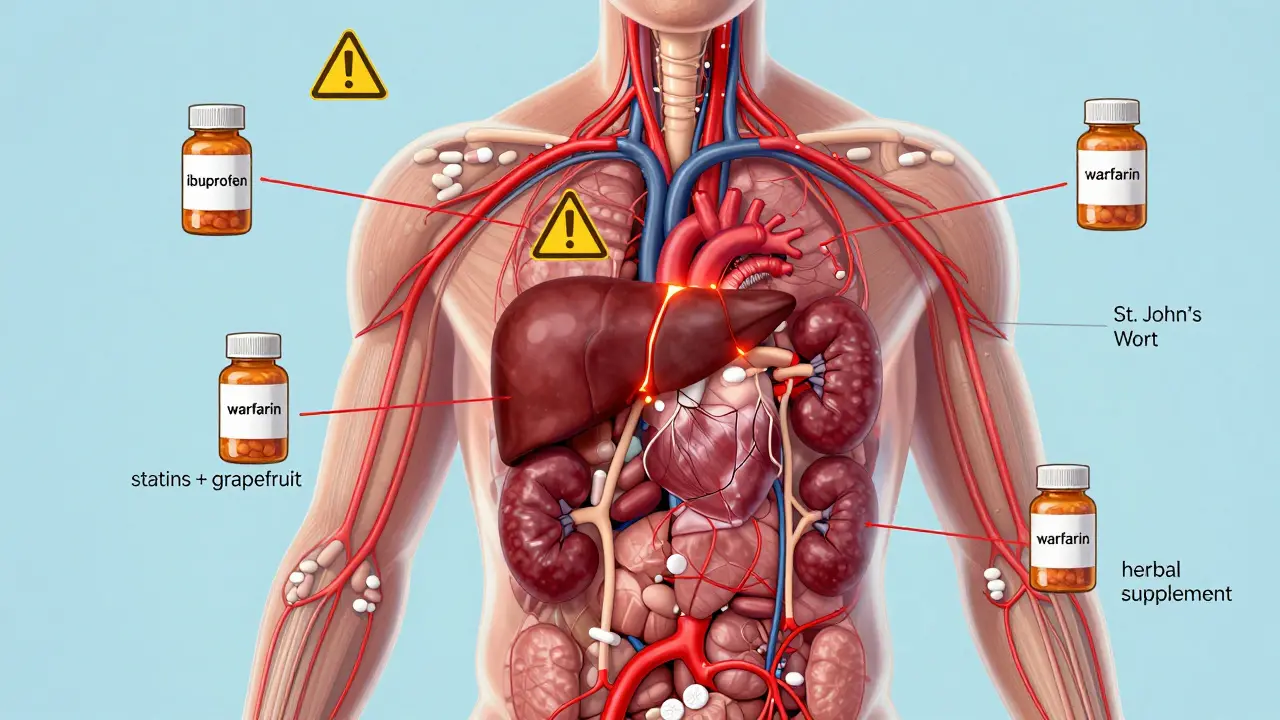
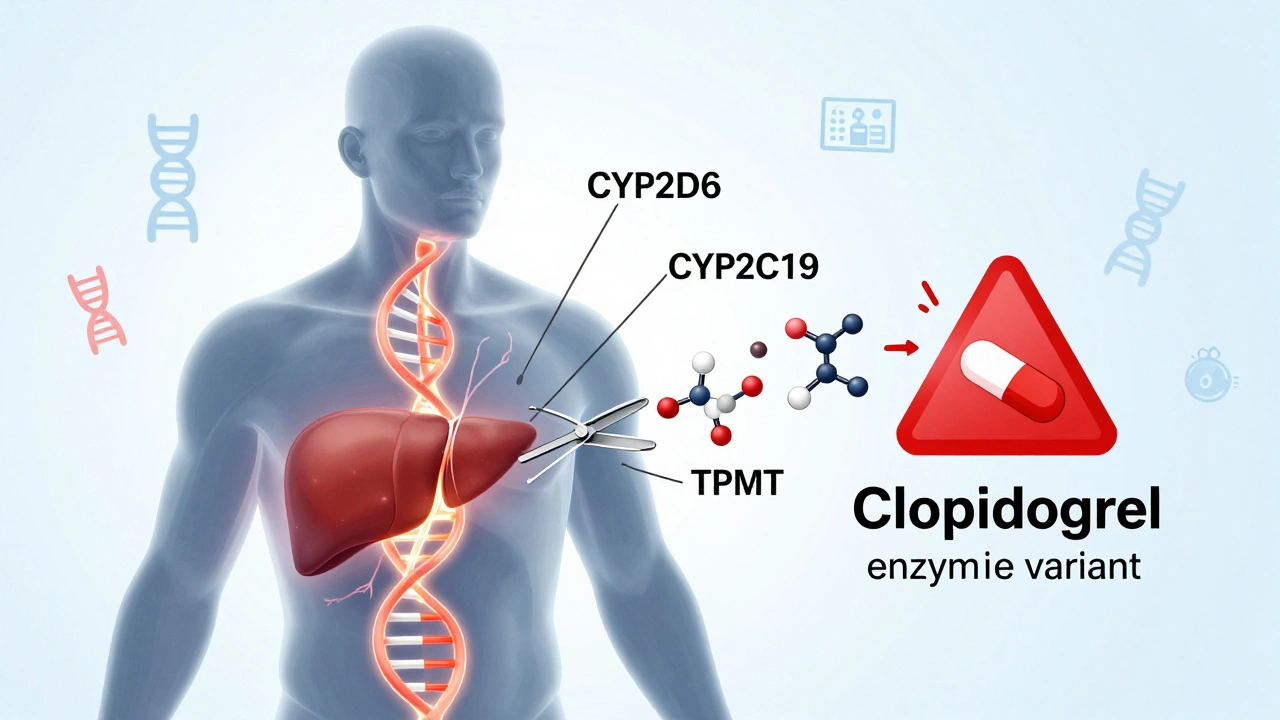
Adverse drug reactions don’t happen in a vacuum. They’re tied to how drugs are made, how they’re prescribed, and how our bodies respond. Generic medication safety, the idea that a copy of a drug must perform just like the original to avoid harm is a big part of this. Two drugs might have the same active ingredient, but if the fillers, coatings, or absorption rates are different, your body might react differently. That’s why bioequivalence studies matter—and why pharmacists are trained to watch for sudden changes when a patient switches brands. Even something as simple as a new diet or a different time of day taking your pill can change how your body handles the drug and trigger an ADR.
Some reactions are obvious—itching, swelling, dizziness. Others hide in plain sight: a sudden drop in energy, unexplained bruising, or confusion in an older adult. If you’ve ever thought, "This doesn’t feel right," and your doctor brushed it off, you’re not alone. But those gut feelings often lead to the discovery of new ADRs. The more people report, the better the system gets. This collection of posts dives into real-world cases—from how liver patients react to new meds, to why certain drugs need stricter safety rules, to how pharmacists are on the front lines spotting trouble before it spreads. You’ll find stories about what went wrong, how it was fixed, and what you can do to protect yourself. No jargon. No fluff. Just what matters when your health is on the line.