Biosimilars: What They Are, How They Compare to Biologics, and Where to Find Them
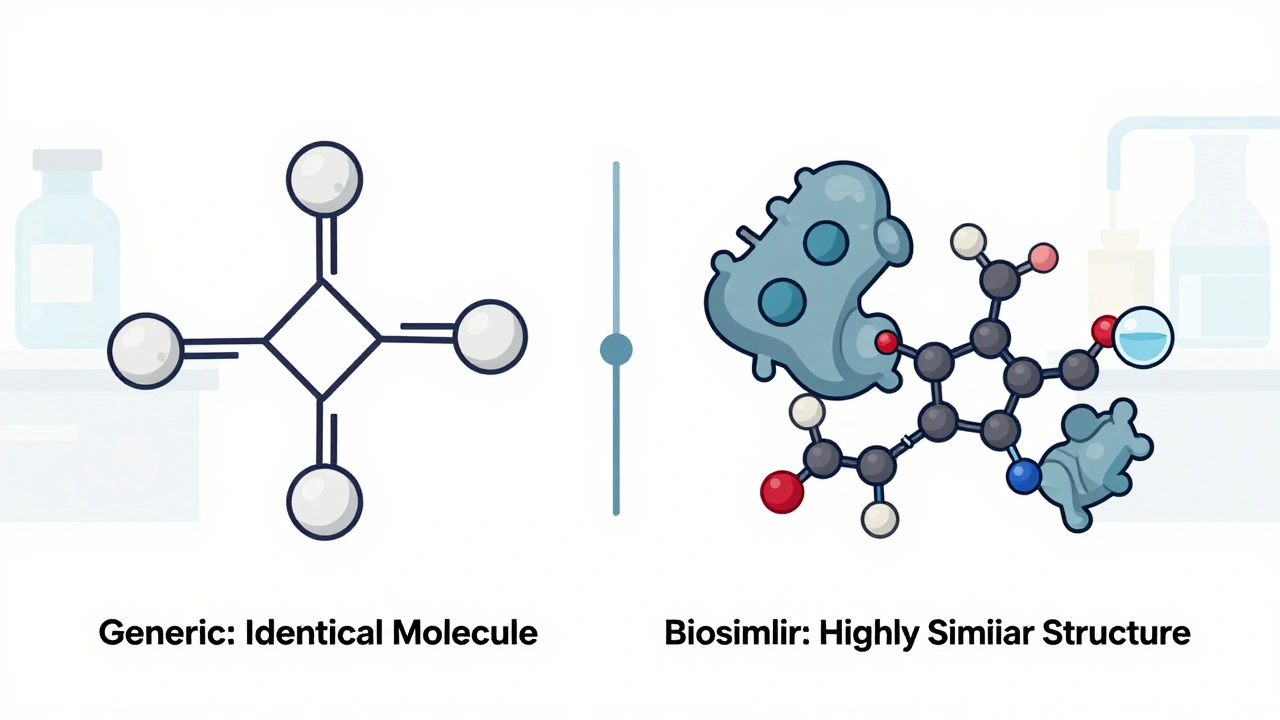
When you hear biosimilars, highly similar versions of complex biologic drugs that are not exact copies but approved to have no clinically meaningful differences in safety or effectiveness. Also known as biologic generics, they are changing how patients access life-saving treatments for conditions like rheumatoid arthritis, cancer, and diabetes. Unlike regular generic pills, which are simple chemical copies, biosimilars are made from living cells—think of them as close cousins, not twins, of the original biologic drug. They’re not cheaper because they’re weaker; they’re cheaper because manufacturers don’t have to repeat the decade-long, billion-dollar research that created the original.
Biologics, the original drugs these are based on, are made from proteins, antibodies, or other molecules grown in living systems. Drugs like Humira, Enbrel, and Remicade fall into this category. These treatments work wonders but often cost over $10,000 a year. That’s where biosimilars, FDA-approved alternatives that match the original in structure, function, and clinical outcome come in. They go through rigorous testing to prove they work the same way, with the same risks and benefits. You won’t get a different result—you’ll get the same result at a fraction of the price. In fact, biosimilars can cut costs by 15% to 35%, sometimes even more.
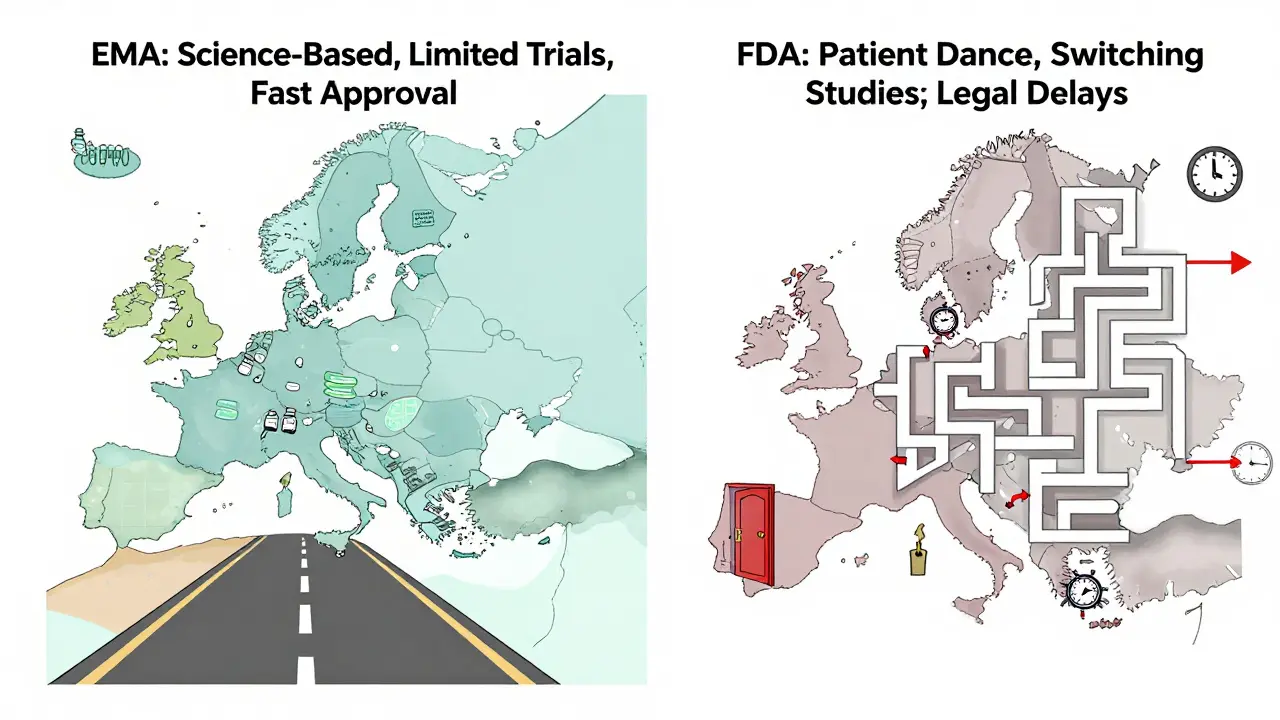
Not all biologics have biosimilars yet—but many do, and more are coming. For example, if you’re taking adalimumab (Humira), you might now be prescribed a biosimilar like Amjevita or Cyltezo. If you’re on insulin glargine (Lantus), you could be using Semglee or Basaglar instead. These aren’t experimental—they’re widely used across the U.S. and Europe, with millions of patients already switching safely. The FDA, the U.S. agency that approves drugs and ensures they meet strict safety and effectiveness standards has cleared dozens of biosimilars, and they’re monitored just like the originals.
Some doctors still hesitate to switch patients, not because biosimilars are risky, but because they’re unfamiliar with them—or because patients are worried about change. But studies show no drop in effectiveness or increase in side effects when switching. Many insurance plans now push biosimilars first because they save money without sacrificing care. If you’re on a biologic and paying out of pocket, asking your doctor about a biosimilar could save you thousands.
What you’ll find below are real, practical guides on how biosimilars fit into everyday treatment. You’ll see how they compare to the originals, what to ask your doctor, where to find them at lower prices, and how they stack up against other cost-saving options like direct-to-consumer pharmacies. Whether you’re managing autoimmune disease, diabetes, or another chronic condition, these posts give you the facts you need to make smarter, cheaper choices without guessing.